Jan 3 2020
On extremely cold days, the atmospheric water vapor can directly change into solid ice and deposit a thin layer of ice on surfaces like a car windshield or windowpane. While this process is quite common, for many years, the details behind this phenomenon have been challenging for chemists and physicists to understand.
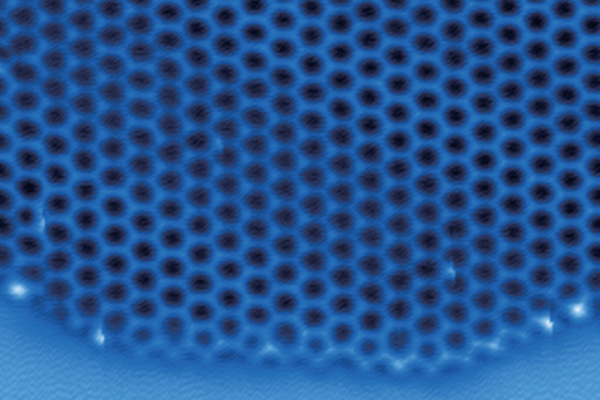 An international team of scientists, including atmospheric chemists from Penn, describe the first-ever visualization of the atomic structure of two-dimensional ice as it formed. Image Credit: Joseph Francisco.
An international team of scientists, including atmospheric chemists from Penn, describe the first-ever visualization of the atomic structure of two-dimensional ice as it formed. Image Credit: Joseph Francisco.
Now, an international research team has elucidated the world’s first visualization of the atomic structure of two-dimensional (2D) ice as it forms. The study has been published in the Nature journal.
Insights gained from the study outcomes—powered by computer simulations that motivated experimental work—may someday help to design materials that make it simpler and less expensive to remove ice.
One of the things that I find very exciting is that this challenges the traditional view of how ice grows.
Joseph S. Francisco, Study Author and Atmospheric Chemist, University of Pennsylvania
“Knowing the structure is very important,” added Chongqin Zhu, study coauthor and a postdoctoral fellow in Francisco’s team who directed most of the study’s computational work. “Low-dimensional water is ubiquitous in nature and plays a critical role in an incredibly broad spectrum of sciences, including materials science, chemistry, biology, and atmospheric science.”
Zhu continued, “It also has practical significance. For example, removing ice is critical when it comes to things like wind turbines, which cannot function when they are covered in ice. If we understand the interaction between water and surfaces, then we might be able to develop new materials to make this ice removal easier.”
In the recent past, Francisco’s laboratory has mainly focused on analyzing the behavior of water, and ice in particular, at the interface of solid surfaces.
Whatever the researchers have learned about the structures and growth mechanisms of ice in this framework helps them to figure out the behavior of ice in more challenging situations, for example, during interactions with atmospheric water vapor and other chemicals.
We’re interested in the chemistry of ice at the transition with the gas phase, as that's relevant to the reactions that are happening in our atmosphere.
Joseph S. Francisco, Study Author and Atmospheric Chemist, University of Pennsylvania
To interpret the fundamental principles of the growth of ice, scientists have ventured into this field of study by analyzing 2D structures—ice layers that have a thickness of just several water molecules.
In earlier studies of 2D ice, Francisco, Zhu, and collaborators used computational simulations and techniques and demonstrated that ice grows in a different way based on whether a surface attracts or repels water, and also the structure of that specific surface.
In the latest study, the researchers pursued real-world confirmation of their computational simulations and collaborated with a Peking University team to see if images of 2D ice could be obtained.
With the help of super-powerful atomic force microscopy, the Peking University team converted the feedback into images of nanoscale resolution. A mechanical probe is used for the atomic force microscopy to “feel” the material being analyzed.
Atomic force microscopy can capture structural data without causing any major disruption to the material itself. This enables the researchers to detect even unstable intermediate structures that emerged at the time of the formation of ice.
Almost all ice that occurs naturally on Earth is referred to as hexagonal ice because of its six-sided structure. That is the reason why a six-fold symmetry is exhibited by all snowflakes. A single plane of hexagonal ice has a structure analogous to that of 2D ice and can terminate in two kinds of edges—that is, “armchair” or “zigzag.” This plane of natural ice often terminates with zigzag edges.
But when ice was grown in two dimensions, the scientists found that the growth pattern was very different. For the first time, the new study demonstrates that it is possible to stabilize the armchair edges and also shows that their growth follows an innovative reaction pathway.
“This is a totally different mechanism from what was known,” added Zhu.
While the patterns of zigzag growth were earlier assumed to have only six-membered rings of water molecules, the atomic force microscopy as well as Zhu’s calculations demonstrated an intermediate stage in which five-membered rings exist.
According to the scientists, this outcome can help describe the experimental observations reported in the PNAS study in 2017. This study discovered that ice can grow in two different ways on a surface, based on that surface’s properties.
The methods utilized in this study provide an understanding of the upcoming design of materials that are favorable for removing ice. They are also relevant for exploring the growth of a huge range of 2D materials beyond 2D ice, thus paving the way for observing the dynamics and structure of low-dimensional matter.
According to Jeffrey Saven, a chemist and professor in Penn Arts & Sciences, the association between the theorists in Francisco’s team and their collaborators in China reminded a fable that he learned from a mentor during his training days. Saven was not directly involved in the latest study.
“An experimentalist is talking with theorists about data collected in the lab. The mediocre theorist says, ‘I can't really explain your data’. The good theorist says, ‘I have a theory that fits your data’. The great theorist says, ‘That’s interesting, but here is the experiment you should be doing and why’.”
To further develop this successful association, Zhu, Francisco, and their collaborators are undertaking experimental and theoretical work to address the gaps relating to how 2D ice develops into three dimensions.
“The two-dimensional work is fundamental to laying the background,” stated Francisco. “And having the calculations verified by experiments is so good, because that allows us to go back to the calculations and take the next bold step toward three dimensions.”
Looking for features of three-dimensional ice will be the next step, and should be very important in looking for applications of this work.
Chongqin Zhu, Study Coauthor and Postdoctoral Fellow, University of Pennsylvania
Joseph S. Francisco is President’s Distinguished Professor in the Department of Earth and Environmental Science, and has a secondary appointment in the Department of Chemistry at the University of Pennsylvania’s School of Arts and Sciences.
Chongqin Zhu is a postdoctoral fellow in the Department of Earth and Environmental Science at the University of Pennsylvania’s School of Arts and Sciences.
Francisco and Zhu’s study coauthors were Peking University’s Runze Ma, Duanyun Cao, Ye Tian, Jinbo Peng, Jing Guo, Ji Chen, Xin-Zheng Li, Li-Mei Xu, En-Ge Wang, and Ying Jiang; and Xiao Cheng Zeng from the University of Nebraska-Lincoln.
The research was supported by the National Key R&D Program (grants 2016YFA0300901, 2017YFA0205003, and 2015CB856801), National Natural Science Foundation of China (grants 11888101, 11634001, 21725302, and 11525520), Strategic Priority Research Program of the Chinese Academy of Science (Grant XDB28000000), Beijing Municipal Science & Technology Commission, and U.S. National Science Foundation (Grant 1665324).