Sep 9 2020
Theoretically, lithium-sulfur batteries (LSBs) have a much greater charge density compared to lithium-ion batteries. Moreover, they are much more economical because of the abundance and affordable price of sulfur.
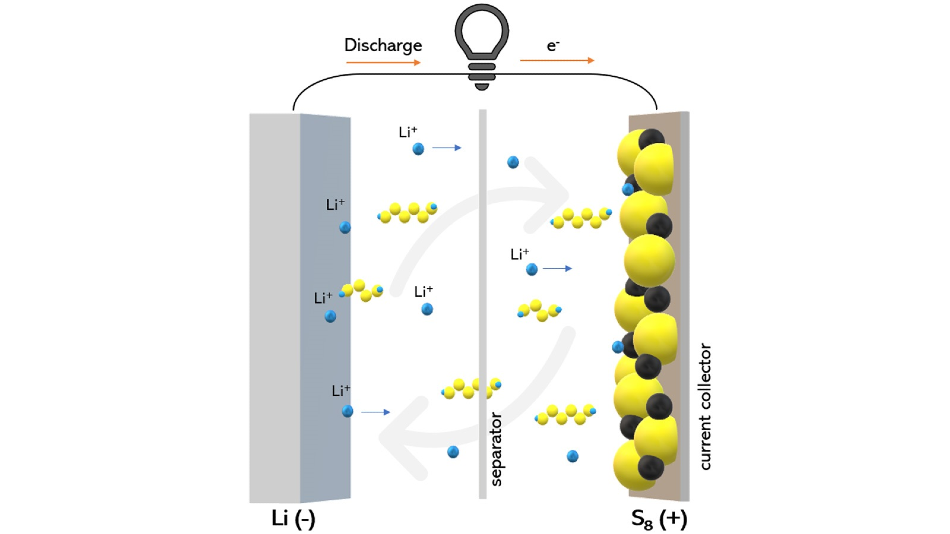 Scientists were able to observe how a certain type of electrolyte material can reduce the shuttling of polysulfide compounds (shown as yellow and blue chains) that impairs the battery’s performance. Image Credit: Wikipedia / Creative Commons.
Scientists were able to observe how a certain type of electrolyte material can reduce the shuttling of polysulfide compounds (shown as yellow and blue chains) that impairs the battery’s performance. Image Credit: Wikipedia / Creative Commons.
In combination, these two factors show that LSBs could be a potential alternative to lithium-ion batteries. But in reality, two major problems have hampered LSBs—low energy efficiency and poor service life.
In a study published in Advanced Energy Materials, scientists from the Argonne National Laboratory of the U.S. Department of Energy (DOE), in collaboration with the National Research Foundation of Korea, have been reviewing how the potential of the sulfur host materials to draw charged particles towards it, or polarity, and its capability to transmit current, or conductivity, can be helpful to overcome these problems and lead to greater feasibility of long-life LSBs.
The lower service life of LSBs can be attributed to a process called “polysulfide shuttle.”
The shuttle is responsible for leaching material from the cathode of the battery. This removal of material degrades the cathode and results in a decreased life span for the battery.
Khalil Amine, Senior Materials Group Leader and Distinguished Fellow, Argonne National Laboratory
When sulfur and lithium react with each other, the polysulfide intermediaries formed at the time of the process tend to dissolve away from the cathode.
In the case of LSBs, it is highly challenging to eliminate the sulfur material from the cathode due to the polysulfide shuttle, and the sulfur itself is extremely insulative, rendering it considerably less conductive.
It’s the combined issues of dissolution and lack of conductivity that present significant obstacles to the development of long-life LSBs.
Gui-Liang Xu, Assistant Chemist, Argonne National Laboratory
Previous studies in the field had demonstrated that the addition of carbon to the cathode structure can help overcome the conductivity problems. However, there is still a debate over what can be done to significantly enhance the performance of the LSBs—fixing the conductivity or the polarity of the cathode.
Amine and his colleagues endeavored to create two different cathode structures to deal with this problem. In one cathode, polar but nonconductive mesoporous silica was used, whereas, in the other one, conductive but nonpolar mesoporous carbon was used.
The two cathodes were designed to be exact replicas of one another apart from the use of either silica or carbon. This way, we could determine whether a more polar cathode or a more conductive cathode improved the longevity of the battery.
Khalil Amine, Senior Materials Group Leader and Distinguished Fellow, Argonne National Laboratory
The researchers employed electrochemical and physical probes to verify whether the findings of their work pointed out that the polar, nonconductive cathode worked well.
“We found that under varying cycling rates and loadings, even at high current densities, the polar, silica-based cathode showed much greater performance than the nonpolar, carbon-based cathode,” added Xu.
Amine further noted that “the stronger interaction between the polysulfides and the silica immobilizes these intermediaries,” thereby reducing the impact of the polysulfide shuttle and extending the service life of the battery. “Our work shows that the key to enhancing the long-term stability of LSBs is enhancing the polarity and enhancing the interactions with the polysulfides at the cathode. If you can stabilize the sulfur, then you can stabilize the battery.”
Using the innovative cathode structure, Amine and his colleagues have shown the practical strength of LSBs and demonstrated that they can serve as viable and low-cost alternatives to lithium-ion batteries.
This study was financially supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office (VTO) Battery Materials Research (BMR) Program; and the National Research Foundation (NRF) of Korea.
Journal Reference
Lee, B.-J., et al. (2020) Revisiting the Role of Conductivity and Polarity of Host Materials for Long-Life Lithium-Sulfur Battery. Advanced Energy Materials. doi.org/10.1002/aenm.201903934.