Dec 15 2020
Low-cost rechargeable batteries form the core of almost all portable electronic devices, which have increasingly become prevalent in day-to-day modern life.
 The higher capacity of this new hard carbon electrode material means that a 19% increase in energy density by weight is possible in sodium-ion batteries compared with lithium-ion batteries. Image Credit: Shinichi Komaba from Tokyo University of Science.
The higher capacity of this new hard carbon electrode material means that a 19% increase in energy density by weight is possible in sodium-ion batteries compared with lithium-ion batteries. Image Credit: Shinichi Komaba from Tokyo University of Science.
Besides this, rechargeable batteries also serve as crucial components in several eco-friendly technologies, like electric systems and cars that harvest renewable energy. Rechargeable batteries are also major enablers of numerous medical instruments and facilitate studies in many different fields as the energy source of cameras and electronic sensors. So, it is no wonder that plenty of batteries occupy the top position because of their excellent performance across the board with regard to stability, capacity, charging time and price.
But lithium and other expensive and minor metals, such as copper and cobalt, are not part of the most abundantly available materials on the Earth’s crust and the rising demand for these metals will soon create supply problems worldwide.
Professor Shinichi Komaba and collaborators from the Tokyo University of Science in Japan have been trying to overcome this worsening problem by designing rechargeable batteries through alternative and more abundant materials.
In a new study, the researchers identified an energy-efficient technique to create a new carbon-based material specifically for sodium-ion batteries. The study was published in the Angewandte Chemie International Edition.
In addition to Professor Komaba, the research group also included Ms Azusa Kamiyama and Associate Professor Kei Kubota from Tokyo University of Science, Drs Yong Youn and Yoshitaka Tateyama from the National Institute for Materials Science in Japan and Associate Professor Kazuma Gotoh from Okayama University in Japan.
The new study targeted the production of hard carbon—an extremely porous material that acts as the negative electrode of rechargeable batteries—by using magnesium oxide (MgO) as an inorganic template of nano-sized pores within the hard carbon.
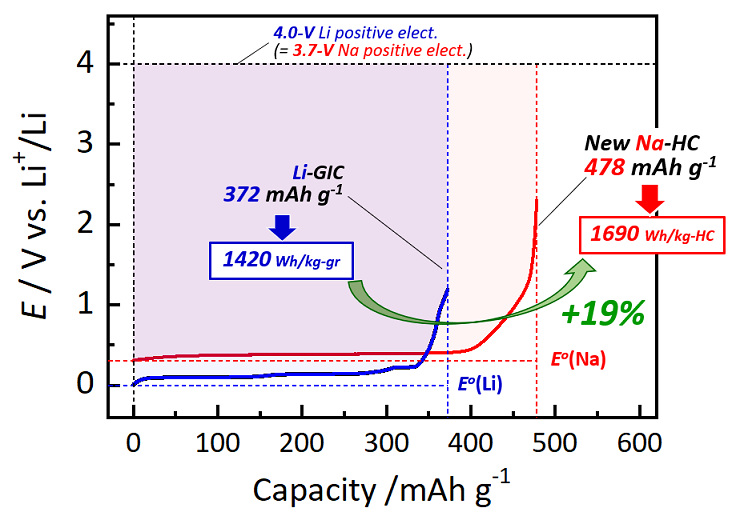
The team investigated another method for combining the ingredients of the MgO template to accurately adjust the nanostructure of the ensuing hard carbon electrode. Following numerous theoretical and experimental studies, the researchers described the optimal manufacturing conditions and ingredients to create hard carbon that has a capacity of 478 mAh/g; this capacity is the highest to be ever reported in a material of this type.
Until now, the capacity of carbon-based negative electrode materials for sodium-ion batteries was mostly around 300 to 350 mAh/g. Though values near 438 mAh/g have been reported, those materials require heat treatment at extremely high temperatures above 1900°C. In contrast, we employed heat treatment at only 1500 °C, a relatively low temperature.
Shinichi Komaba, Professor, Tokyo University of Science
Evidently, lower temperatures translate to lower energy expenditure, which also implies less environmental impact and lower cost.
This recently designed hard carbon electrode material indeed has an extraordinary capacity, considerably exceeding that of graphite (372 mAh/g), which is presently used in lithium-ion batteries as the negative electrode material.
Furthermore, while a sodium-ion battery integrated with this hard carbon negative electrode would theoretically work at a lower voltage difference of 0.3 V when compared to a regular lithium-ion battery, the higher capacity of the former battery would result in a relatively greater energy density by weight (that is, 1600 Wh/kg against 1430 Wh/kg), leading to more than 19% increase in energy density.
Happy about the outcomes and looking forward to the future, Professor Komaba added, “Our study proves that it is possible to realize high-energy sodium-ion batteries, overturning the common belief that lithium-ion batteries have a higher energy density. The hard carbon with extremely high capacity that we developed has opened a door towards the design of new sodium-storing materials.”
Nevertheless, additional studies will be needed to validate that the recommended material truly provides better input-output properties, lifetime and low-temperature operation in real sodium-ion batteries. If this theory works out, the next generation of rechargeable batteries may soon become a reality.
Journal Reference:
Kamiyama, A., et al. (2020) MgO-Template Synthesis of Extremely High Capacity Hard Carbon for Na-Ion Battery. Angewandte Chemie International Edition. doi.org/10.1002/anie.202013951.