Phosphate glass is a multipurpose compound that has gained interest for its use in fuel cells and as biomaterials for administering therapeutic ions.
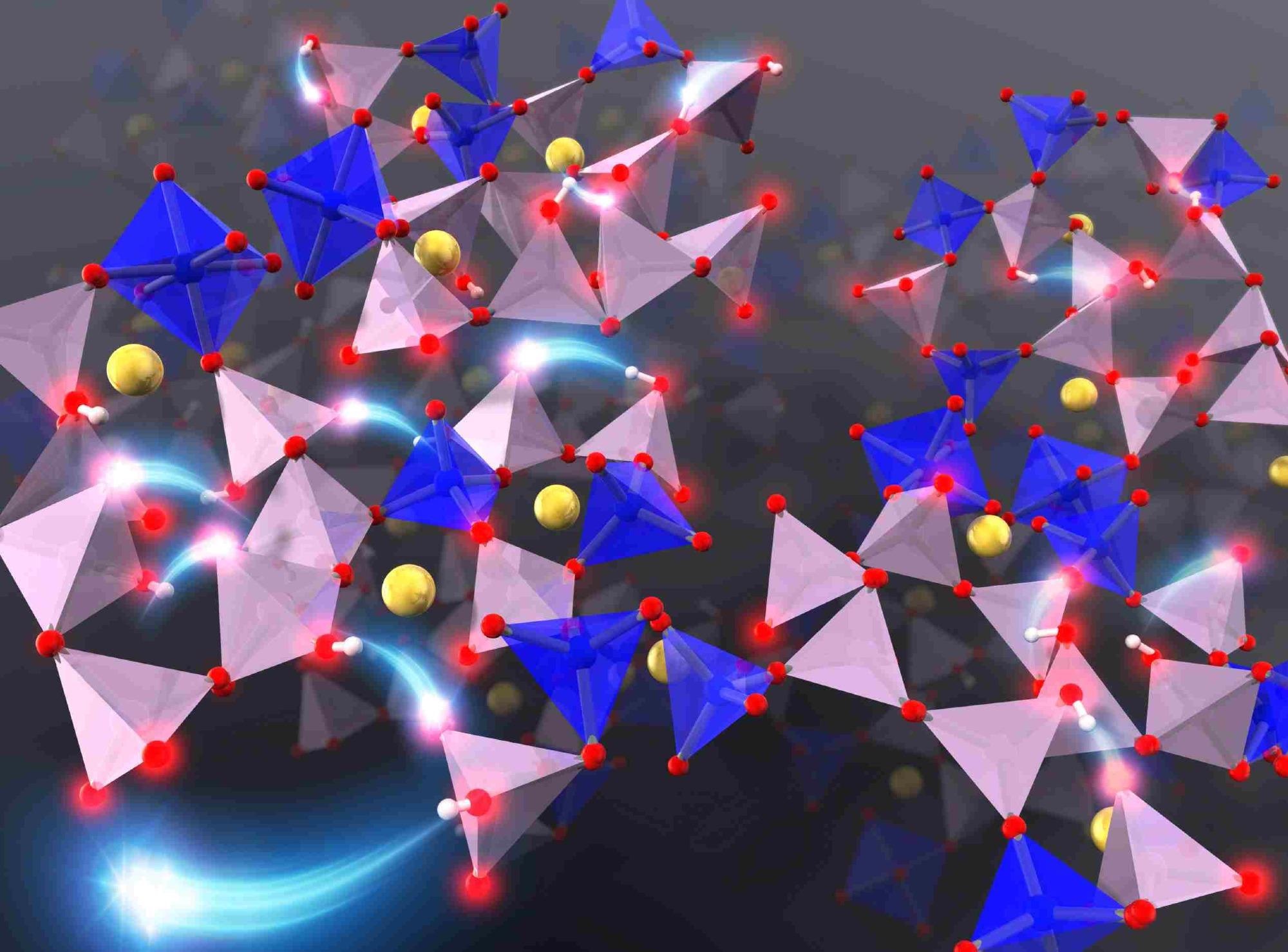 Investigating the microscopic diffusion mechanism of protons and sodium ions in phosphate glasses via first-principles molecular dynamics simulation indicates the key role of the morphology of the phosphate network structure on the diffusion of ions. Image Credit: Tomoyuki Tamura, Nagoya Institute of Technology.
Investigating the microscopic diffusion mechanism of protons and sodium ions in phosphate glasses via first-principles molecular dynamics simulation indicates the key role of the morphology of the phosphate network structure on the diffusion of ions. Image Credit: Tomoyuki Tamura, Nagoya Institute of Technology.
P2O5—the compound that constitutes the structural network of phosphate glass, is composed of phosphorus, an element that can take different bonding configurations when combined with oxygen.
The physicochemical properties vital for the real-world applications of phosphate glass—for example, the hydration reaction determining how rapidly a phosphate glass-based biomaterial can dissolve within the body—relies on the diffusion of ions into the glass. Therefore, to enhance the physicochemical properties of phosphate glasses, it is important to understand the relationship between the structure and ion diffusion.
However, examining such interactions at the atomic level is hard, prompting researchers to look for an appropriate process to highlight the details of the ion diffusion process.
In a recent study, a group of scientists from Nagoya Institute of Technology, headed by Dr. Tomoyuki Tamura, theoretically decoded the ion diffusion mechanism associated with the hydration reaction process of phosphate glasses. The research has been published in the journal Physical Chemistry Chemical Physics.
In completely connected P2O5-based phosphate glass, three of the oxygen atoms in each phosphate unit are linked to neighboring phosphorous atoms. To analyze the dynamics of ions in the phosphate glass during the hydration process, the scientists employed a model made of phosphates with QP2 and QP3 morphologies, which has two and three bridging oxygens per PO4 tetrahedron, respectively, in addition to six coordinated silicon structures.
The team used a theoretical, computational approach named “first-principles molecular dynamic (MD) simulation” to examine the diffusion of proton and sodium ions into the glass. Dr. Tamura explained the rationale for their unconventional approach.
First-principles MD simulation enabled us to assume the initial stage of water infiltrating and diffusing into silicophosphate glass and elucidate the diffusion of protons and inorganic ions for the first time.
Dr Tomoyuki Tamura, Nagoya Institute of Technology
Based on their observation, the scientists put forth a mechanism where the protons “hop” and are adsorbed onto the non-bridging oxygen or “dangling” oxygen atom of nearby phosphates through hydrogen bonds. But in the phosphate glass model they used, the QP2 phosphate units contributed actively to the diffusion of protons than the QP3 phosphate units.
The researchers identified that the morphology of the phosphate network structure, or the “skeleton” of the glass, highly affects the diffusion of ions. They also observed that in the presence of a sodium ion, the adsorption of a proton onto a QP2 phosphate unit lessened the electrostatic interaction between sodium and oxygen ions, inducing the chain diffusion of sodium ions.
There is a growing need for new biomaterials for effective treatment and prevention, and phosphate glasses stand ready to meet this growing demand. A huge proportion of the population, including younger and older adults, suffer from diseases related to muscle and bone weaknesses.
Water-soluble silicophosphate glass is a promising candidate for supplying drugs or inorganic ions that promote tissue regeneration, and our study takes the research in glass technology one step nearer towards realizing the goal.
Dr Tomoyuki Tamura, Nagoya Institute of Technology
The novel perceptions of the scientists are bound to have intense real-world impact and can lead to advancements in the research on fuel cells and bioresorbable materials.
Journal Reference:
Takada, K., et al. (2021) Diffusion of protons and sodium ions in silicophosphate glasses: insight based on first-principles molecular dynamic simulations. Physical Chemistry Chemical Physics. doi.org/10.1039/D1CP01646F.