Although it is compelling to recycle carbon dioxide (CO2), particularly via conversion to methane (CH4), anthropogenic CO2 emissions are still increasing.
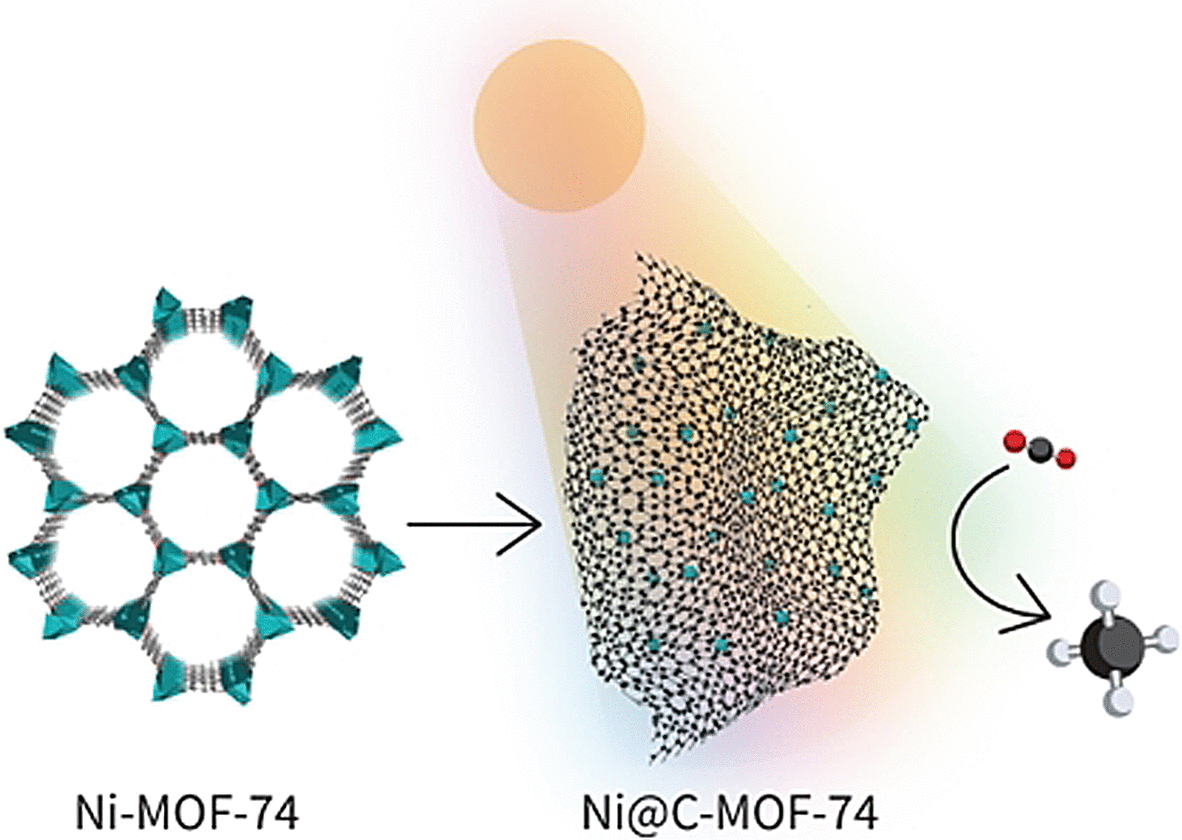 Image Credit: © Wiley-VCH, 'Angewandte Chemie' and a link to the original article.- https://onlinelibrary.wiley.com/doi/10.1002/anie.202111854.
Image Credit: © Wiley-VCH, 'Angewandte Chemie' and a link to the original article.- https://onlinelibrary.wiley.com/doi/10.1002/anie.202111854.
A suitable process for this conversion is photothermal methanation, wherein CO2 and hydrogen are catalytically turned into CH4 and water upon solar irradiation. In the journal Angewandte Chemie, a group of researchers has recently described the synthesis of a very active, stable, nickel–carbon catalyst for this reaction.
The researchers directed by Luis Garzón-Tovar and Jorge Gascon at King Abdullah University of Science and Technology (Thuwal, Saudi Arabia) were seeking to find an efficient, cost-effective catalyst for the photothermal methanation of CO2. Photothermal catalysis is dependent on the integration of light-driven and thermal chemical processes.
Contrary to pure photocatalysis, it has the benefit of facilitating longer wavelength light in the visible and IR regions of the spectrum to add to stimulating the reaction.
In place of precious metals, they aimed to base the new catalyst on a copious, economical metal and chose to work with a high load of nickel nanoparticles on a carbon-based support. Carbon materials are very favorable supports for photothermal catalysis as they absorb a wide spectrum of light, are extremely efficient in changing light into heat energy, and possess a large surface area.
The researchers used a nickel-containing metal-organic framework (Ni-MOF-74) as their primary material for creating the catalyst. Regulated pyrolysis of this material at 600°C turned out to be ideal. The Ni-MOF-74 decomposes to develop uniform finely spread nickel nanoparticles set in in a porous graphitic carbon matrix.
The resulting material, termed Ni@C, showed a high rate of conversion and high selectivity for methanation under artificial IR, visible, UV, light. In a continuous procedure in a flow-type reactor, the catalyst’s efficiency remained stable over 12 hours.
To showcase the practical application of this process, an experiment was conducted outside, under natural sunlight, establishing the potential of this new catalyst to convert CO2 to CH4 using solar energy.
Journal Reference:
Khan, I. S., et al. (2021) An Efficient Metal–Organic Framework-Derived Nickel Catalyst for the Light Driven Methanation of CO2. Angewandte Chemie. doi.org/10.1002/anie.202111854.