Tissue engineering offers excellent potential in various medical applications, representing a potential solution to help replace failed organs or missing tissues. The success of a tissue engineering technique is highly linked to its ability to evolve appropriate scaffolds that are able to accurately mimic the extracellular matrix. Significant research has been undertaken into the development of suitable scaffold structures, including thin films, nanoparticles, composites, and also hydrogels.
Hydrogels, in particular, represent attractive options for this task. These biomaterials are made up of three-dimensional, hydrophilic cross-linked polymeric networks that absorb and hold significant amounts of biological fluids. Hydrogels are also degradable, meaning they are ideal starting points for tissue growth and even cell transplants.
It is necessary to adapt the characteristics of these hydrogels based on their target application. Specific cross-linking methods are frequently used to achieve this customization.
The review opts to focus on proteins due to their structural and functional characteristics, highlighting that proteins represent an excellent option for hydrogel fabrication due to their natural biodegradability, biocompatibility, and their reduced tendency to trigger inflammatory responses in surrounding tissues.
Proteins can be cross-linked by aggregating these into a gel network or using chemical coupling or covalent cross-linking methods.
The review of the use of protein-based hydrogels in tissue generation highlights a number of opportunities, barriers, and shortfalls in the existing work in this area, providing a valuable roadmap for future developments in this field.
The authors note a gap in the use of protein-based hydrogels in clinical applications, despite their vast potential in this area. Limitations around the traditional manufacture of protein-based hydrogels are outlined, not least that while proteins themselves are generally composed of biocompatible long chains, the use of solvents in the generation of protein-based hydrogels can have an adverse impact on cell viability.
The authors point out that more work is needed in this particular area to comprehensively assess the inflammatory and immune responses of protein-based hydrogels in order to identify optimal solvent choices and ensure maximum biocompatibility.
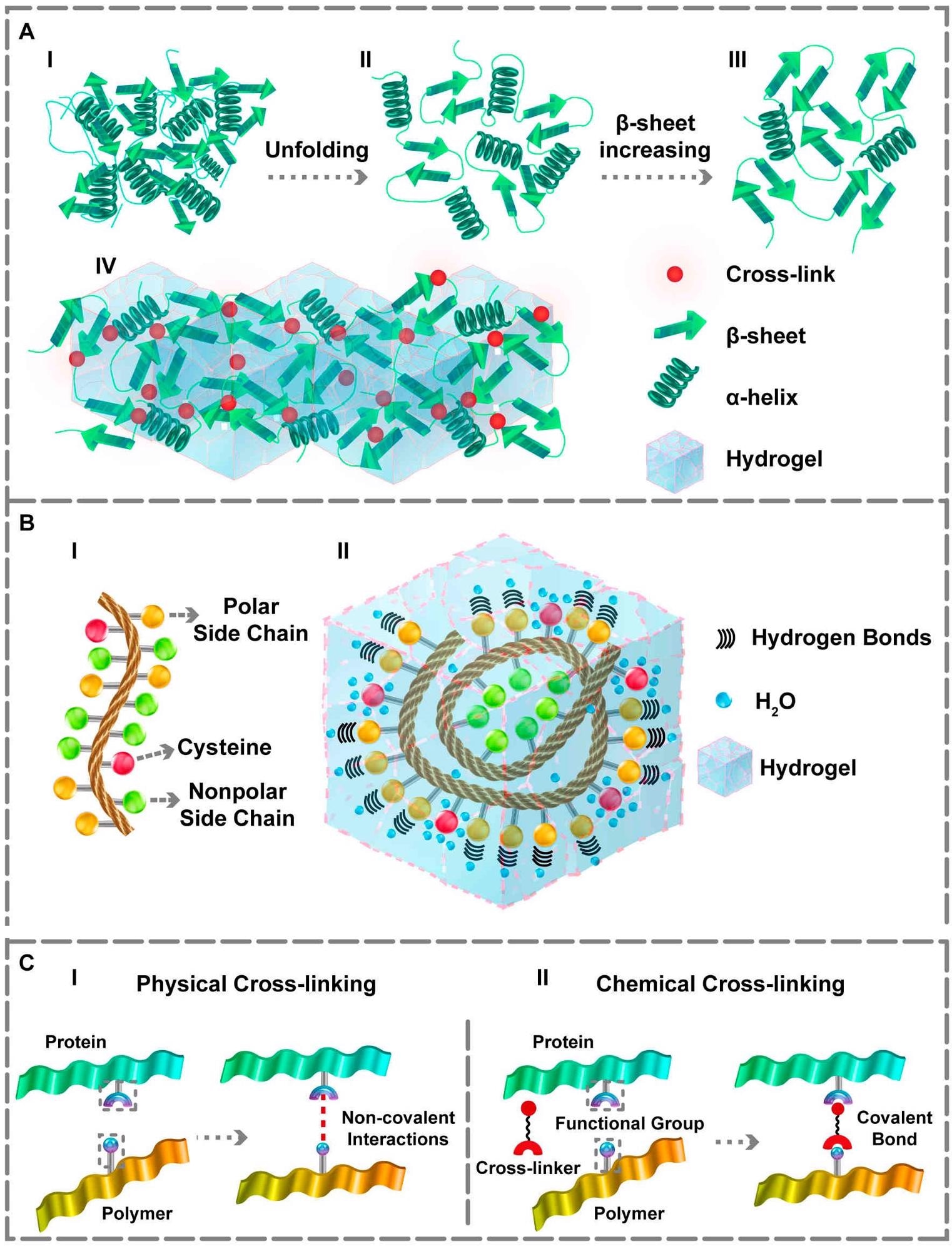
The protein’s unfolding and structural changes and its interaction within the hydrogel matrix. (A) (I, II) Conformational changes from the third to second structure. (III, IV) Increasing the β‐sheet content which forms the desired gel matrix. (B) (I) Amino acids sequence. (II) Representation of hydrogen bonds within the hydrogel in the presence of polar side chains and the water‐holding capacity of cysteine residue. The side chains of the polar amino acids make proper hydrogen bonds. Additionally, cysteine residue can form better hydrogels owing to the –SH group that assists waterholding capacity. (C) (I) Physical and (II) Chemical cross‐linking approaches for obtaining PBHs gel matrix. Image Credit: Davari N et al., Polymers
It was also highlighted that a key factor in improving protein-based hydrogels’ biocompatibility is their resilience and ability to adapt to fluctuations in the body’s physiological conditions, for example, slight changes in temperature or pH that commonly occur within the human body. It is anticipated that this issue will attract considerable attention as the field of protein-based hydrogels develops.
One interesting development highlighted in the review paper is the use of in situ forming hydrogels, which exist in a liquid state at ambient temperature. These can be injected into the body, where they experience a phase tradition. The paper notes that there is excellent potential for in situ hydrogels to be combined with protein-based therapeutics or cancer drugs and that their minimal invasiveness could yield good results in terms of improving drugs’ bioavailability, reducing side-effects, and enhancing overall patient compliance.
A number of barriers exist to fully leveraging in situ hydrogels in this way – for example, limited research on biodegradability in tumor microenvironments and the potential for immunogenicity – but work is ongoing to address these issues and improve clinical applicability.
While the review highlights several gaps in research and barriers to leveraging the full potential of protein-based hydrogels in tissue engineering applications, this work represents an important step towards addressing these issues and advancing the field as a whole.
Most notably, the authors highlight the importance of tailoring the development of protein-based hydrogels to their target application and ensuring that the desired clinical outcomes remain at the center of the protein-based hydrogels’ development process.
The ability to achieve these outcomes will depend on factors such as protein structure, interactions between proteins, delivery strategies, biocompatibility, and the development of optimized polymeric matrices within the hydrogels themselves.
References
Davari N, Bakhtiary N, Khajehmohammadi M, Sarkari S, Tolabi H, Ghorbani F, Ghalandari B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers. 2022; 14(5):986. https://www.mdpi.com/2073-4360/14/5/986
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.