CDs are fluorescent carbon-based materials that emit fluorescence when excited. Due to this property, CDs have found many chemicals as well as biological applications. Researchers are particularly attracted to its surface functional group, which is the main origin of fluorescence emission on excitation. Besides yielding good fluorescence, CDs are highly soluble, non-toxic, biocompatible, and are photochemically stable.
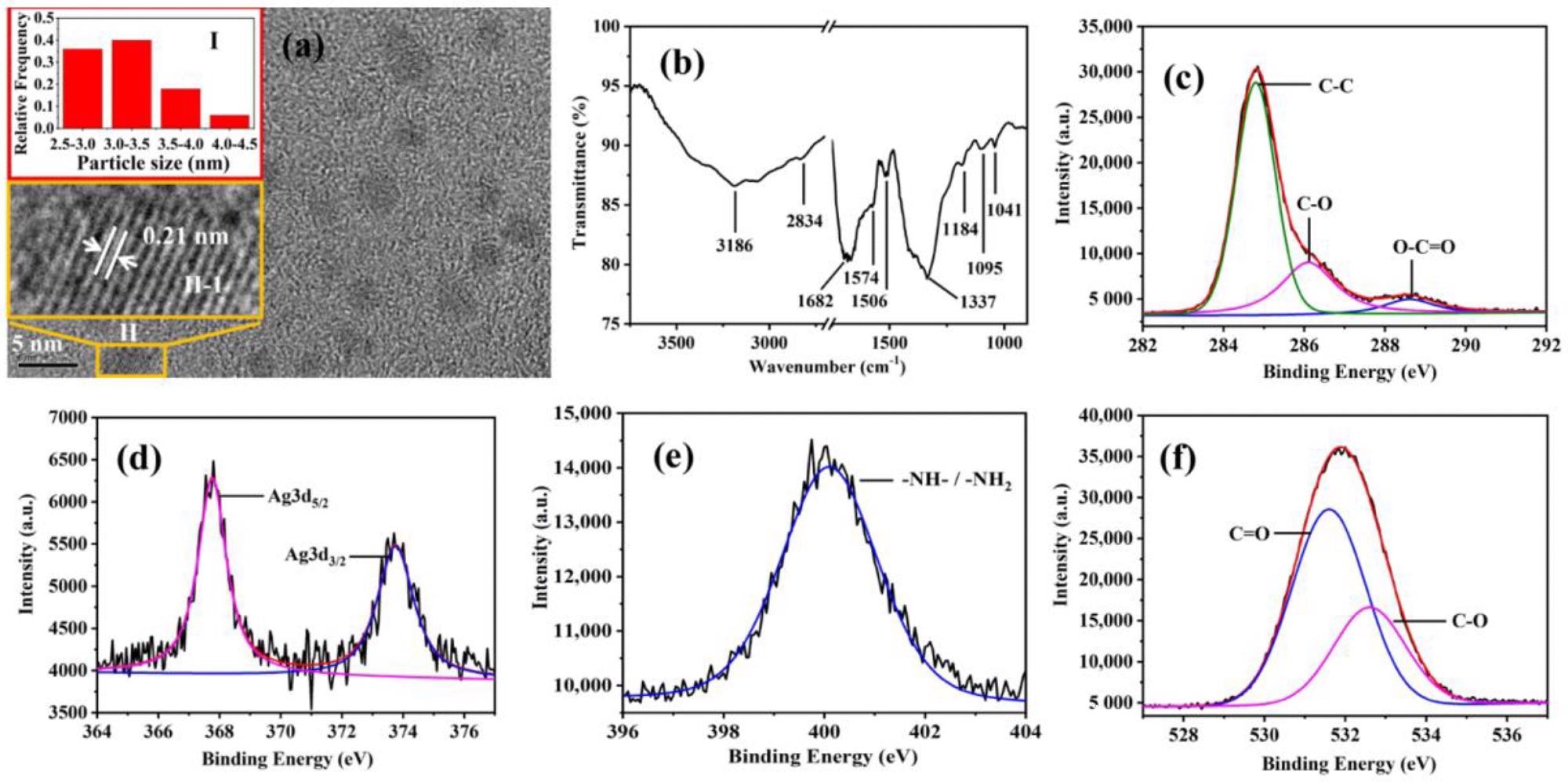
(a) TEM image of Ag-CDs. (Inserts: (I) Particle size distribution histogram of Ag-CDs. (II-1) Magnified image of ”II” Ag-CD.) FT-IR spectrum (b), high-resolution XPS spectra of C1s (c), Ag3d (d), N1s (e), and O1s (f), respectively, of Ag-CDs. Image Credit: Xu, Q.; Li, K.; Wang, P, Materials
Through in-depth research on CD behavior, researchers have found that irrespective of the fabrication method, the origin of fluorescence emission is from special edge states that consist of many carbon atoms and surface carboxyl or carbonyl groups.
Studies also found that shorter wavelength emissions of CDs are independent of excitation and are mainly dependent on the surface amino group states and the longer wavelength emission is caused by the carboxyl and hydroxyl groups.
Some researchers have also observed that metals such as Ag+ greatly enhance the fluorescence of CDs by the chelation of Ag+ with CD surface functional groups. Thus, doping Ag+ directly into CDs during fabrication is the best way to prepare Ag-containing CDs or Ag-CDs. Although thorough research on the effect of pH on Ag-CD fluorescence emission behavior is yet to be done, there is some evidence showing fluorescence is regulated by pH.
The Study
In the present study, a hydrothermal method was applied to prepare Ag-CDs. A solution of folic acid and silver nitrate dissolved in pure water was transferred into the autoclave, followed by 12 hours of heating at 2000C. The suspension was centrifuged for 10 mins at 15000 rpm, filtered three times using a microfiltration membrane, and then was dialyzed for 24 hours. The morphology of the prepared Ag-CDs was studied using transmission electron microscopy (TEM) and the diameter of the particle was measured.
To examine the effect of pH on Ag-CDs, the pH of the Ag-CD solution was adjusted using an aqueous solution of NaOH and H2SO4 at various values ranging from 0.74 to 13.50 and the reversibility of the pH effect was tested between pH values of 12.15 to 2.25. A digital pH meter was used to measure the pH value and a fluorescence spectrophotometer was used to record the emission spectra. In addition, a pH fluorescent test paper was created in the lab to test the ability of Ag-CDs to act as a pH fluorescent probe.
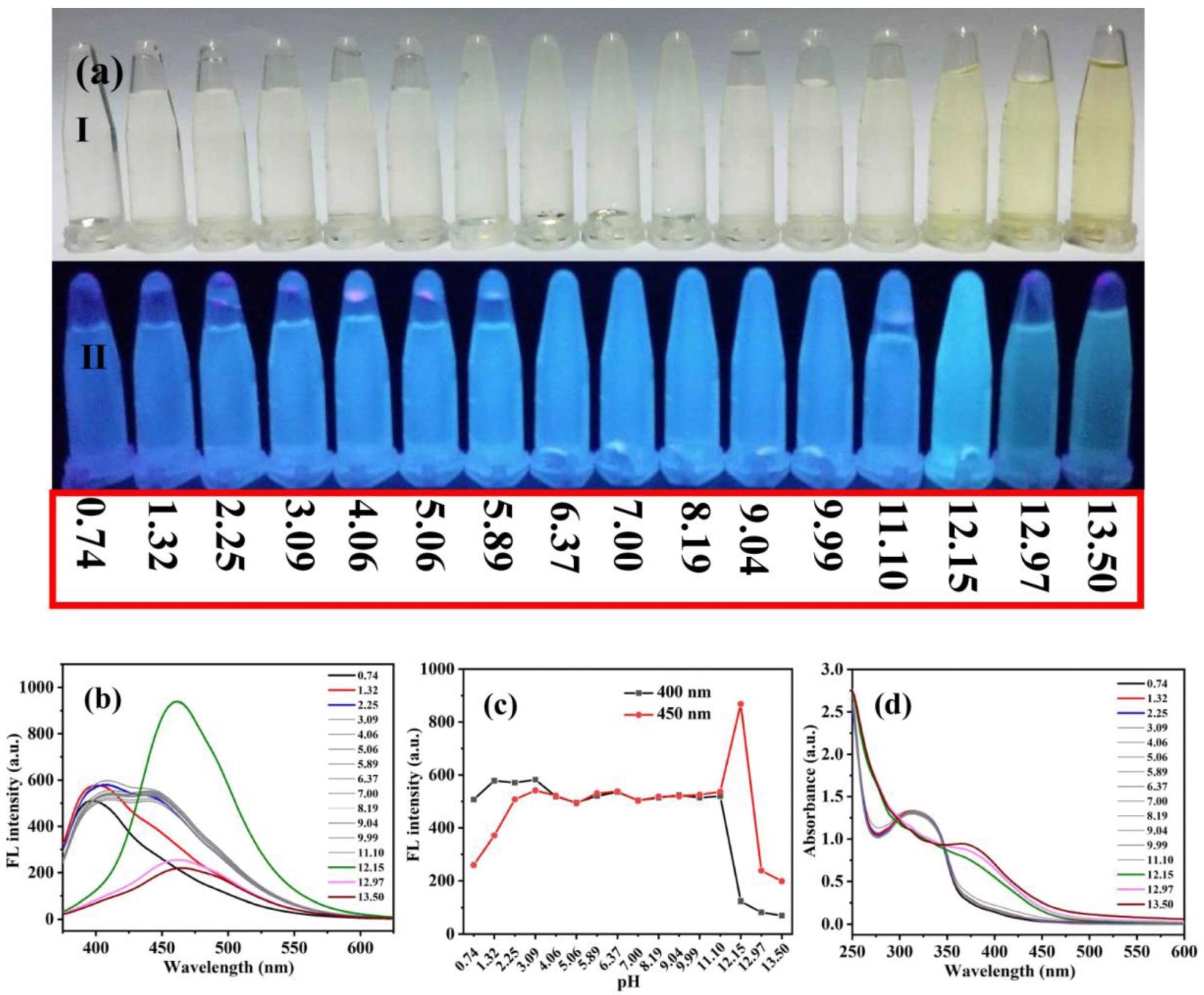
(a) Photographs of Ag-CDs in aqueous solutions at various pH values under daylight (a-I) and UV light (365 nm, a-II). Fluorescent emission spectra at λEX 360 nm (b), fluorescent intensity probed at 400 and 450 nm (c), and absorption spectra (d) of Ag-CDs detected at various pH values. Image Credit: Xu, Q.; Li, K.; Wang, P, Materials
Observation
Fourier transferred infrared absorption spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) composition showed the presence of primary amine, secondary amine, carboxyl groups, and the hydroxyl group at the edge of the carbon backbone.
Weaker and moderate absorption ranges of 350-500 nm and 275-350 nm, respectively, were observed in a neutral solution. Based on the fluorescent excitation spectra, two species of fluorescence were found to exist in Ag-CDs: one showed a fixed electronic structure in the 250-330 nm absorption range and the other was an excitation-dependent species in the 250-500 nm absorption range.
The effect of pH on Ag-CD fluorescence was studied by determining their fluorescence emission spectra and ultraviolet-visible (UV-Vis) absorption at pH values ranging from 0.74 to 13.50. Under 365 nm UV light, all solutions exhibited blue fluorescence. Significantly weaker emission was observed at extremely acidic or extreme alkaline conditions; at a pH value of 12.15, the brightest fluorescent was observed. However, the emission was the same at all other pH values.
The results demonstrated good reversibility of the prepared Ag-CDs by varying the pH values, which implies its potential to be a fluorescent sensor at a broad range of pH. Moreover, the pH fluorescent test paper experiments showed that when soaked in ultrapure water the test paper showed the brightest fluorescence at pH = 12.20, indicating that it can be used as a sensitive pH-sensor in strongly alkaline conditions.
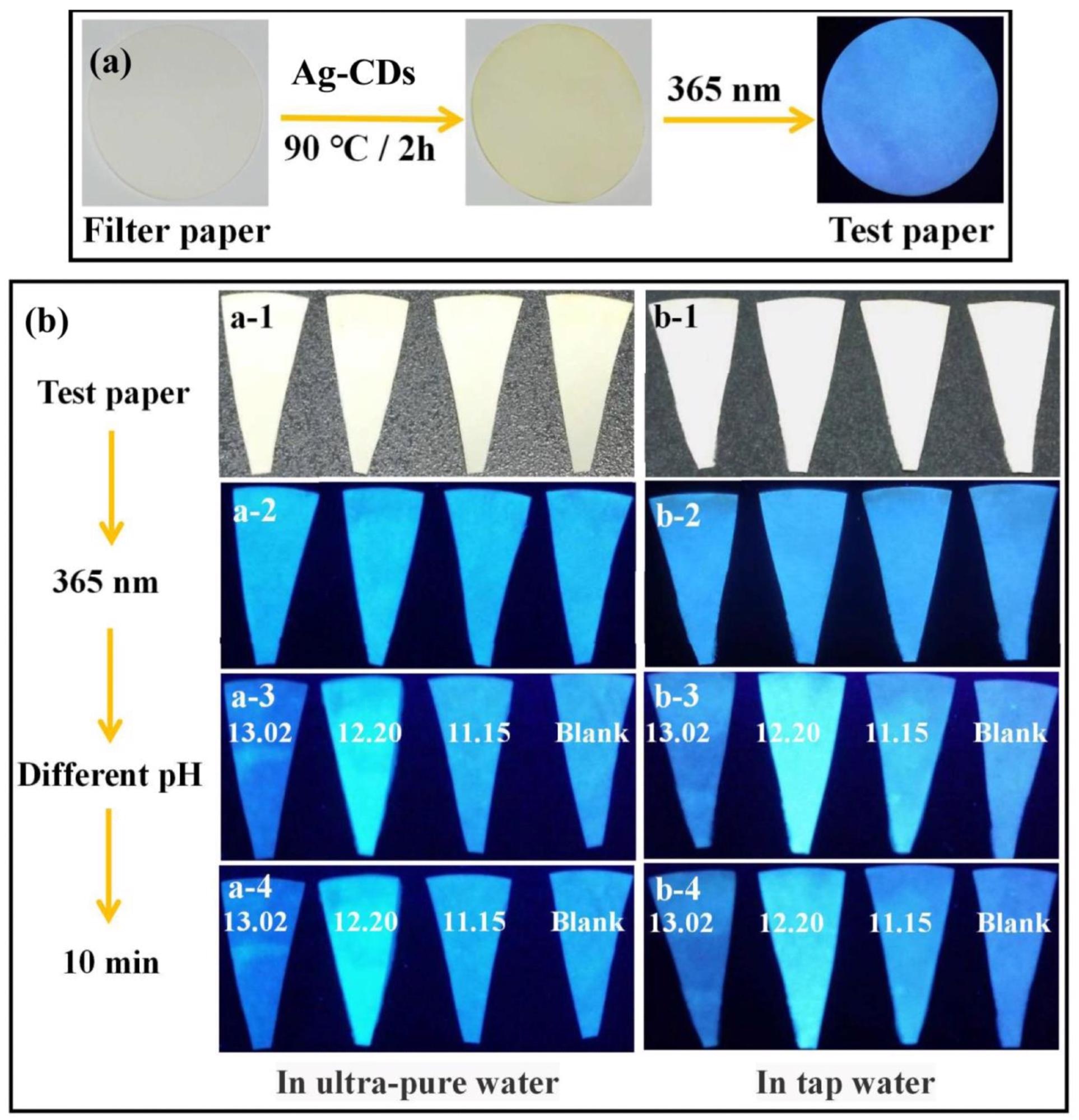
(a) The pH fluorescent test paper was prepared by filter paper and Ag-CDs (excited at 365 nm). (b) The pH fluorescent test paper for different pH sensing (13.02, 12.20, 11.15, and Blank) in ultrapure water and tap water for 10 min (excited at 365 nm). Image Credit: Xu, Q.; Li, K.; Wang, P, Materials
Conclusion
To summarize, a hydrothermal method was used to couple CDs with Ag+ in this work. Using a broad range of pH values and various spectroscopic methods, the structural mechanism of the Ag-CDs was investigated. XPS results showed that the fluorescence of Ag-CDs is regulated by pH.
The most striking effect of pH on fluorescence emission was observed at the pH value of 12.15. The pH fluorescent test paper and the fluorescent reversibility test showed that Ag-CDs prepared by the researchers could be developed into a fluorescent sensor, especially at extremely alkaline conditions. This research opens up avenues for the development of such pH-regulated fluorescent sensors based on metal ion-doped CDs that can be regulated with pH.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.