In recent times, iodine is increasingly clubbed with zinc anodes to make sustainable zinc–iodine (Zn–I2) batteries (ZIBs) because of its high redox capability (I2/I-, 0.62 V vs. regular hydrogen electrode) and large hypothetical capacity (211 mAh g-1).
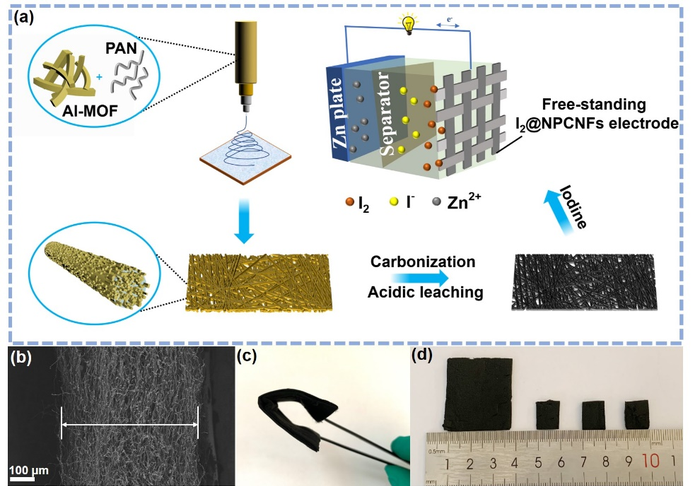 (a) Schematic illustration for the synthesis process of I2@NPCNFs-800 to fabricate zinc-iodine battery. (b) Cross-section SEM image of NPCNFs-800. (c,d) Photographs of flexible and freestanding NPCNFs-800 electrode. Image Credit: ©Science China Press
(a) Schematic illustration for the synthesis process of I2@NPCNFs-800 to fabricate zinc-iodine battery. (b) Cross-section SEM image of NPCNFs-800. (c,d) Photographs of flexible and freestanding NPCNFs-800 electrode. Image Credit: ©Science China Press
More significantly, iodine’s conversion reaction with high energy efficiency on a cathodic chamber bestows ZIBs with unique benefits over metal-ion batteries. Regrettably, ZIBs batteries experience a low utilization ratio of active source, insulating iodine and the shuttle effect, causing rapid capacity decay and unwanted self-discharging problems.
Numerous types of porous carbons to restrain iodine and boost electrochemical kinetics have been developed. In recent times, metal-organic framework (MOF) has been seen as a favorable precursor to create heteroatoms-doped porous carbon materials because of their controllable pore size, large surface area and modifiable structures.
To additionally enable modern energy storage and prolong the lifetime of batteries, the rational design of a self-supported, versatile and binder-free cathode is extremely vital for ZIBs. Electrospinning is a facile and flexible technology to create a free-standing three-dimensional (3D) interconnected network.
Thus, the combination of the innate benefits of the MOF and the exclusive features of electrospinning is practical to fabricate versatile fibrous membranes for improving reversible iodine accommodation.
A while ago, Dr. Jin-Tao Zhang’s group demonstrated a facile technique for the creation of self-supported N-doped porous carbon nanofibers (NPCNFs) through a regulated thermal treatment of Al–MOF/ PAN composite fibers made with the electrospinning technique.
Polyacrylonitrile (PAN) bridging among the Al–MOF nanorods is a good way to acquire the incorporated carbon fibers for manufacturing the hierarchically porous carbon network. Making the most from the exclusive porous structure and nitrogen doping, the as-prepared NPCNFs can act as a conductive aid to firmly capture iodine and efficiently hinder its dissolution.
When assessed as cathode materials for ZIBs, the I2@NPCNFs-800 displayed remarkable long-time cycling resilience of 6,000 cycles. The in-situ Raman spectroscopy exposed that NPCNFs offered the relatively stable electrochemical setting for reversible redox conversion.
Before fabricating the nanofibers, highly dispersed and even Al-MOF rod-like were made. Then, the Al-MOF/PAN fibers were fabricated through the electrospinning technique with the precursor solution comprising N, Al-MOF, N-dimethylformamide (DMF), and PAN.
The thermal treatment at 800 °C in N2 and subsequent acidic leaching resulted in the creation of free-standing and versatile porous carbon nanofibers with nitrogen doping (i.e., NPCNFs-800).
Next, iodine was loaded through the melt-diffusion technique and the acquired composite without extra additives was called I2@NPCNFs-800. The I2@NPCNFs-800 film can be directly verified as a free-standing electrode to create ZIBs for accomplishing high reversible capacity and good cycling life.
The scientists investigated the diverse calcination temperatures of the materials (700-900 °C) on the behavior of the zinc-iodine battery. Out of all of them, the carbon nanofiber membrane calcined at 800 °C displayed shuttle inhibition of iodine and more robust adsorption, which exhibited the ideal rate capability, cyclic stability and self-discharge.
Furthermore, the pore size, composition and reaction kinetics of numerous samples calcined at varying temperatures were examined to elucidate the variances in their electrochemical behavior.
In conclusion, the energy storage mechanism of the zinc-iodine battery was exposed by in-situ Raman spectroscopy. The outcomes clearly showed the reversible transformation: I5-↔I3-↔I-.
Moreover, the I 3d XPS spectra was carried out to detect the valence state of iodine species during charge/discharge progression, which also showed a very reversible method and efficient two-step conversion reaction mechanism for the study’s ZIBs.
Journal Reference:
He, Y., et al. (2022) Shapeable carbon fiber networks with hierarchical porous structure for high-performance Zn-I2 batteries. doi.org/10.1007/s11426-021-1177-1.