Carbon materials are increasingly being researched for fields such as electrochemistry and in applications that capture CO2 and reduce the impact of greenhouse gas emissions. Nitrogen is essential for both these applications. Studies on the use of heteroatoms in carbon materials used as electrodes for oxygen reduction reactions have demonstrated quaternary nitrogen and pyridine species are essential.
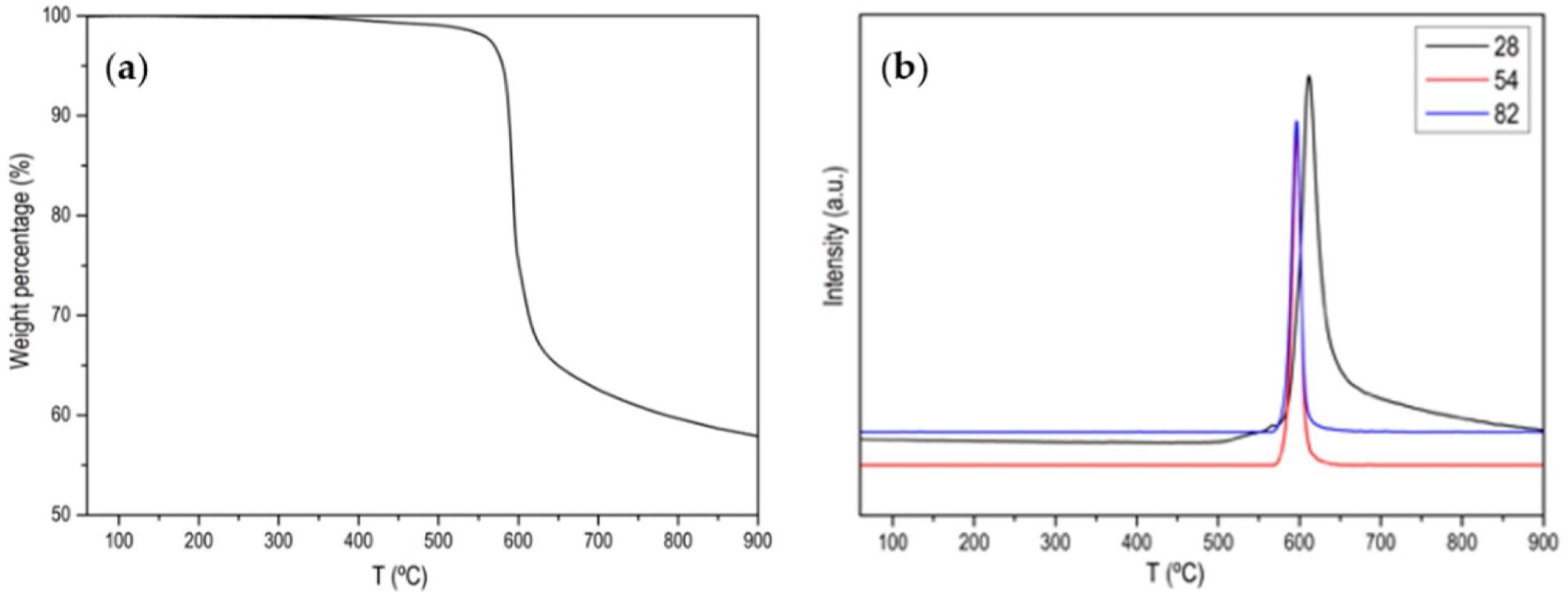
(a) Normalized TG profile of ZIF-67 in Ar atmosphere; (b) MS signal for m/z 28 (N2), m/z 54 and m/z 82 (main 2-Methylimidazole fragmentation). Image Credit: Villalgordo-Hernández, D et al., Materials
Conventional nitrogen doping strategies have only achieved less than 8 at.% incorporation into carbon materials; therefore, new synthetic methods that increase this content are necessary. Studies have so far found contradictory evidence for the role of nitrogen in CO2 capture, and it can be concluded that its presence is necessary, but only if the type of nitrogen that adequately improves capture is determined. Research has indicated that a main factor remains the sample’s microporosity.
Metallic oxygen frameworks are a main competitor of carbon materials in CO2 capture research. These materials exhibit a greater versatility in both surface chemistry and porosity, which are important for capturing carbon dioxide.
However, these materials can display instability when used for applications. UiO-66 has superior stability and can be easily manufactured but possesses low absorption capacity, making it insufficient for CO2 capture. Modifying the linkers, which contain nitrogen, improves this performance, further indicating that nitrogen plays an essential role in carbon dioxide capture.
Some types of these materials, especially zeolitic imidazolate frameworks, have been explored for the generation of carbon frameworks that possess a high nitrogen content. They possess immense stability due to their high decomposition temperature in non-oxidizing atmospheres (around 500oC.) Indeed, several studies have achieved this.
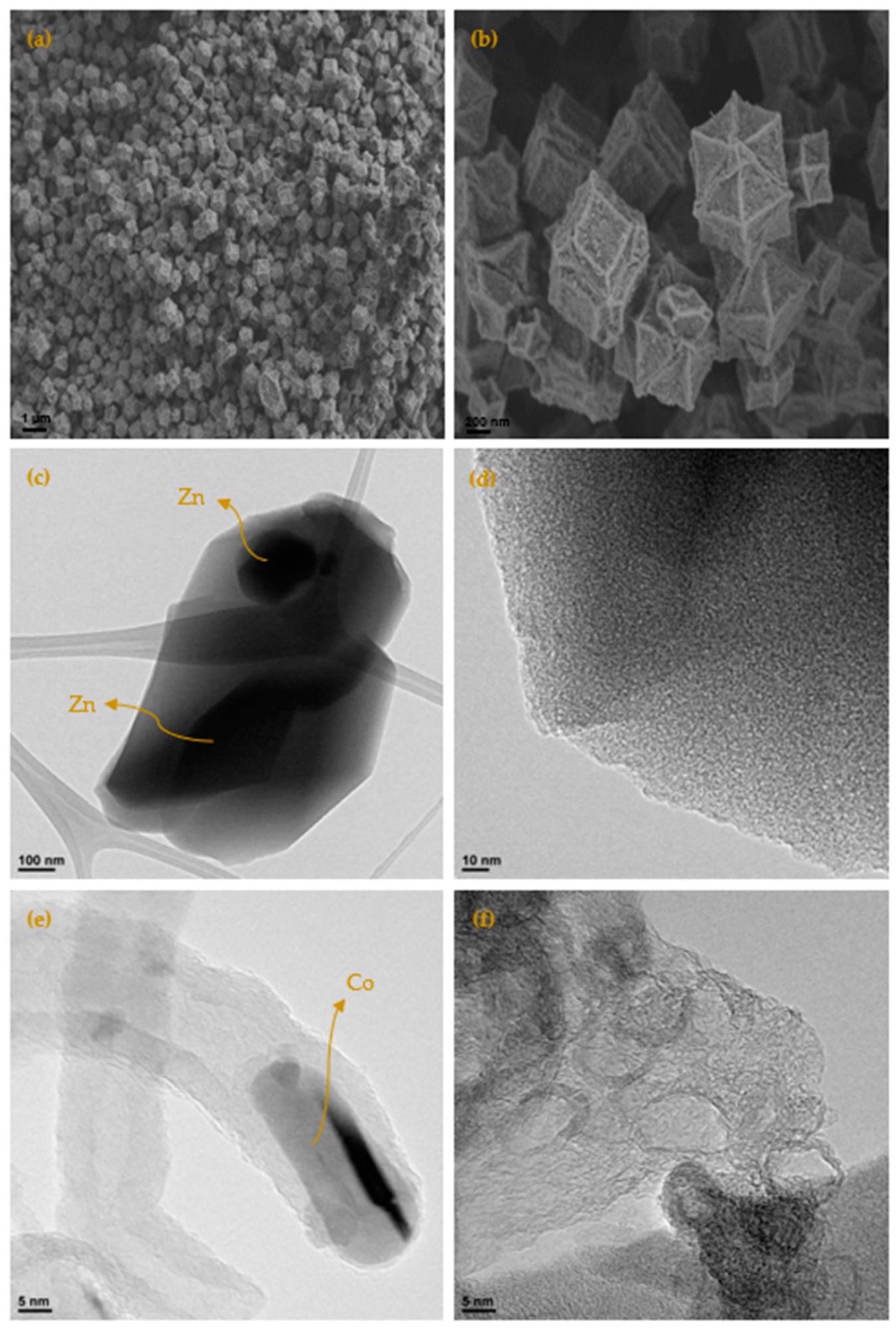
(a) SEM micrograph by means of secondary electrons of the carbonized sample at 700 °C (ZIF67C_700); (b) detail of the micrograph; (c) TEM micrograph of the sample ZIF8C_700, where the turbostratic microstructure of the carbon and the Zn particle can be clearly seen; (d) detail of the micrograph of the turbostratic zone; (e) TEM micrograph of the sample ZIF67C_900, where the formation of a carbon nanotube is shown, with the cobalt particle at one end and the highly ordered carbonaceous structure; (f) TEM micrograph of sample ZIF67C_900_2l, where it is clearly seen that the cobalt particles have been removed leaving the very crystalline structure of the carbon phase. Image Credit: Villalgordo-Hernández, D et al., Materials
The Study
The authors behind the research in Materials have obtained several activated carbon materials from zeolitic imidazolate frameworks. The two frameworks used in the research are ZIF-8 and ZIF-67. Some derivatives have also been used in the research to generate carbon materials. The results of the author’s methods have produced carbon materials with a much higher nitrogen content than conventional methods, at greater than 20 at.%.
The researchers observed that the activated carbon’s nitrogen content decreased with the use of higher temperature treatments. Both types of zeolitic imidazolate framework produced carbons with a superior CO2 absorption capacity, and their adsorption capacity depends on the nitrogen content and the volume of their micropores. Analysis of the materials revealed the presence of pyrrolic structures, pyrimidine, and quaternary nitrogen.
The samples were thoroughly analyzed and categorized by the researchers. Scanning electron microscopy with X-ray microanalysis and transmission electron microscopy were used to study the activated carbon material’s morphology. Porosity of the materials was categorized using a Quantachrome device. CO2 adsorption was analyzed to determine diffusional restrictions, revealing there were none in the samples. Micropore volume was obtained by analysis of diffusional restrictions.
Different zeolitic imidazolate frameworks produced carbon materials with different microstructures. ZIF-8 (zinc) generated carbons with turbostratic structures, whereas ZIF-67 (cobalt) produced graphitic structures. Materials with a better-developed porosity were generated by zinc, and they possessed a slightly higher nitrogen content, making ZIF-8 more suitable for carbon dioxide capture.
Conversely, materials obtained from ZIF-67 are more suitable for electrochemistry due to their high nitrogen content and graphitic structure. The results of the analysis therefore demonstrated that the different metallic oxide frameworks generate carbon structures that can be used for different applications.
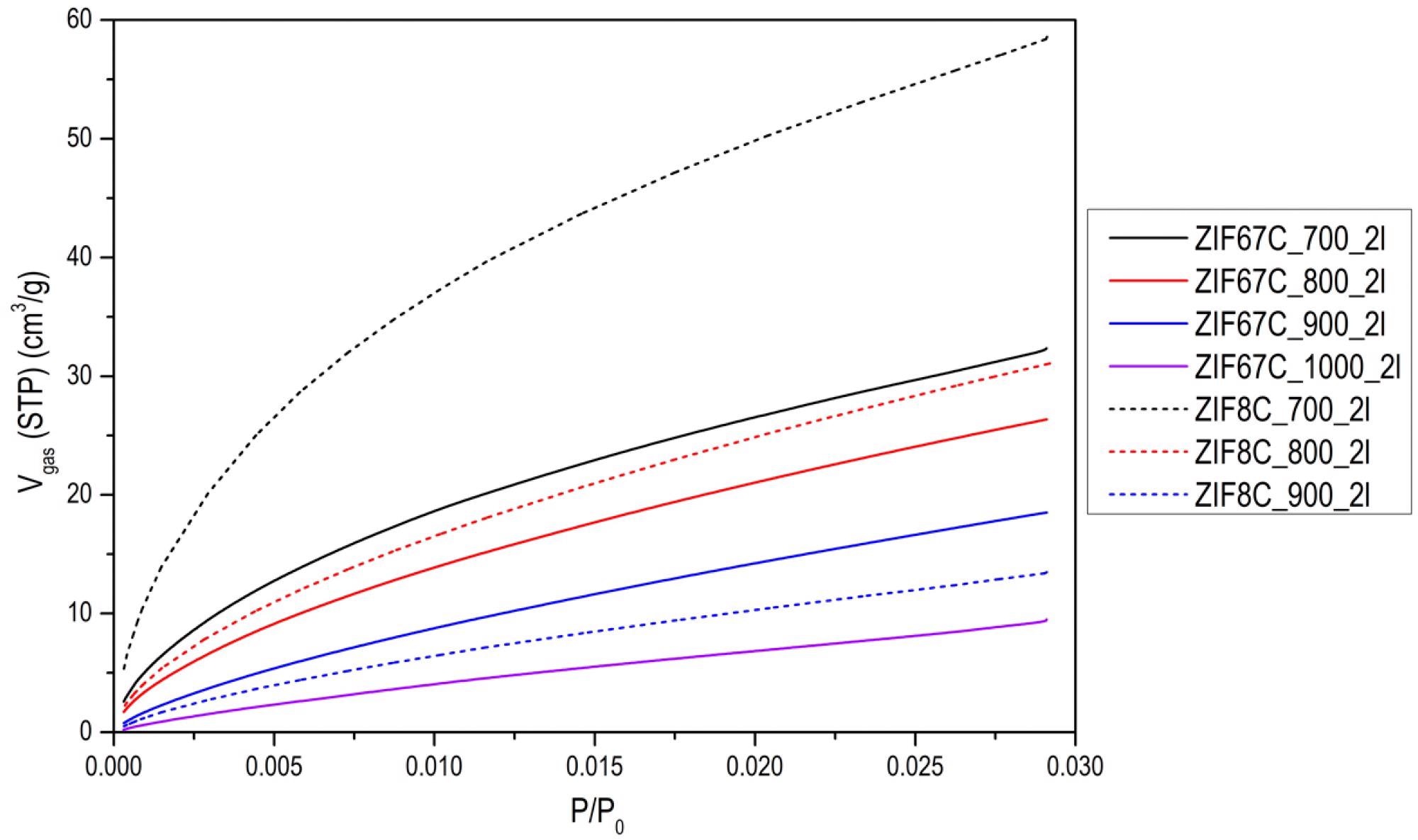
CO2 adsorption isotherms at 0 °C of some of the activated carbons. Image Credit: Villalgordo-Hernández, D et al., Materials
Summary of the Research
The authors have presented a novel synthesis process for activated carbons containing a high nitrogen content by utilizing the stability of metallic oxide frameworks to generate carbons. This research has implications for the fields of electrochemistry, electrocatalysis, and CO2 capture.
Results of the findings demonstrated materials that possessed a nitrogen content exceeding 20 at.%, which is significantly higher than conventional synthesis methods, which typically can only generate carbons with a nitrogen content no higher than 8 at.%, thereby more than doubling the synthesis efficiency. Analysis results indicated good porosity in the prepared activated carbons, with a lack of diffusional restrictions, which, alongside nitrogen content, is essential for CO2 adsorption and capture.
The method presented in the study has enormous potential for renewable technologies, improving the green nature of industries and helping to mitigate the damaging effects of anthropogenic climate change.
Further Reading
Villalgordo-Hernández, D et al. (2022) Manufacture of Carbon Materials with High Nitrogen Content [online] Materials 15(7) 2415 | mdpi.com. Available at: https://www.mdpi.com/1996-1944/15/7/2415
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.