Under the guidance of Professor Jianjun Liu from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences, researchers have recently come up with a high-performance solid electrolyte of Li3Zr2Si2PO12 (LZSP) with space group of C12/c1 (No. 15) from the Na3Zr2Si2PO12 (NZSP) precursor through a method of skeleton-retained cationic exchange.
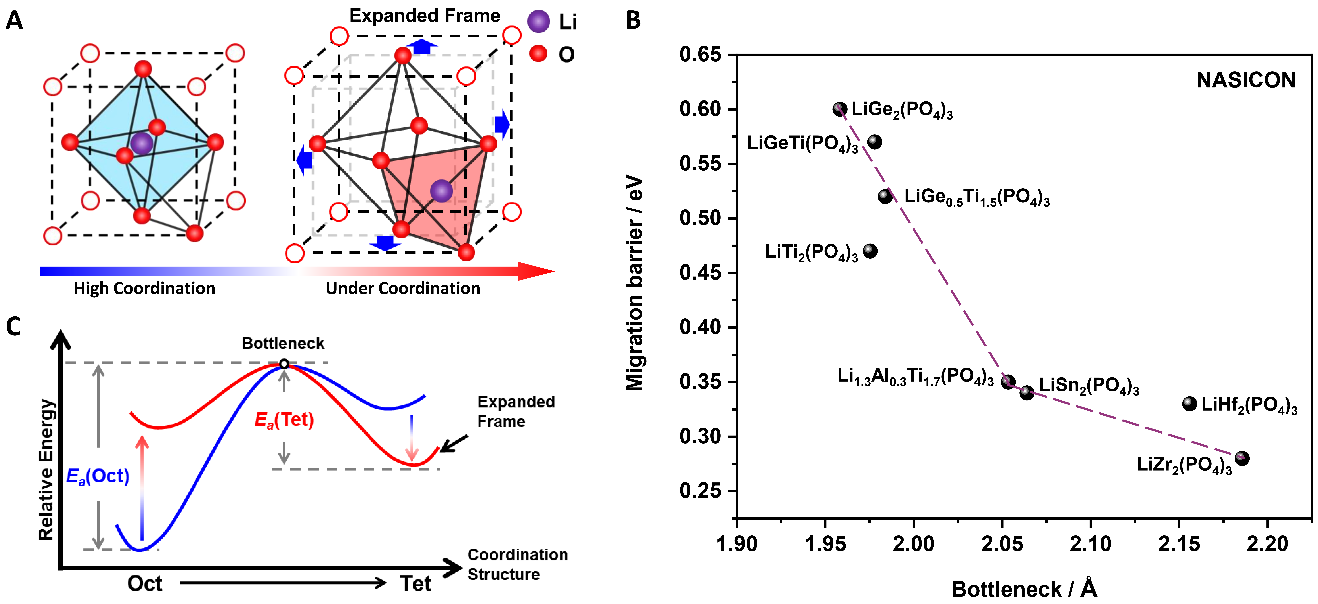 Relationships among coordination modulation, bottleneck, and migration energy barrier. (A) Structural schematic of coordination engineering for the transition between LiO6 hexahedrons and LiO4 tetrahedrons. (B) Migration barrier of Li+ ions from LiO6 hexahedrons to LiO4 adjacent tetrahedrons. (C) Relations between bottleneck and the migration energy barrier of lithium-ion in NASICON-type lithium solid electrolytes. Image Credit: Jianjun Liu.
Relationships among coordination modulation, bottleneck, and migration energy barrier. (A) Structural schematic of coordination engineering for the transition between LiO6 hexahedrons and LiO4 tetrahedrons. (B) Migration barrier of Li+ ions from LiO6 hexahedrons to LiO4 adjacent tetrahedrons. (C) Relations between bottleneck and the migration energy barrier of lithium-ion in NASICON-type lithium solid electrolytes. Image Credit: Jianjun Liu.
This was done in close collaboration with Professor Weiping Tang’s group from the Shanghai Institute of Space Power-Sources.
An experimental and theoretical strategy has been suggested by the researchers to improve the conductivity of solid electrolyte ions. This could be done by relocating diffusion ions to under-coordination sites, which sets the stage for a new approach and the production of new materials. The study outcomes have been reported in the Science Advances journal.
Solid electrolytes are considered crucial materials for enhancing the energy density, safety and reversibility of electrochemical energy storage batteries. Even though few sulfides solid electrolytes, like Li10GeP2S12, execute high ionic conductivity, the low stability against Li metal results in a rapid decrease of ionic conductivity.
The other solid electrolytes, like oxide solid electrolytes with entire stability (chemical, electrochemical, and environmental) display low ionic conductivity, thereby blocking the additional application.
This happens essentially as a result of the narrow bottleneck of lithium-ion from octahedral to tetrahedral occupying. Hence, reaching a high ionic conductivity for oxide solid electrolytes has been a complicated and long-lasting task as a result of the limit of the bottleneck size.
In this study, the scientists suggested a plan of skeleton-retained cationic exchange (Na+→Li+) to synthesize LZSP material in the experiment, and a coordination self-adaptation mechanism was disclosed for Li-ions to engage under-coordination sites and receive the wide bottleneck from NZSP precursor. This plays a major role in improving ionic conductivity, in theory.
Experimental and theoretical validation was carried out for this cationic exchange to make Li-ion occupied in tetrahedral sites to retain structural stability and the migration bottleneck sizes of Li ions are considerably increased as a result of inherited structure from Na-ion solid electrolyte.
Consequently, the synthesized LZSP was illustrated to not only have the highest Li-ion ionic conductivity at room temperature in oxide solid electrolytes but also present significant stability for air and Li metal.
In this study, the suggested technique of skeleton-retained cationic exchange varies from the generally adopted molten salt ion-exchange method, by which phase transformation might occur and lead to low ionic conductivity.
Also, it could be used to generate more solid electrolytes, even wide inorganic materials, by regulating ideal reaction conditions.
Journal Reference:
Zhu, L., et al. (2022) Enhancing ionic conductivity in solid electrolyte by relocating diffusion ions to under-coordination sites. Science Advances. doi.org/10.1126/sciadv.abj7698.