Lithium-ion battery (LIB) technology has advanced dramatically during the last two decades. LIBs have proven their exceptional performance in terms of operating voltage, life cycle, energy density, and self-discharge rate, as well as low volume. Despite LIBs' enticing applicability, constraints such as poor performance at low temperatures, short lifetimes, and, most importantly, the rapid depletion of lithium ore supplies may be a setback for the technology. Many studies have been performed on alternate elements for ion battery technology based on this concept.
Because of their non-toxicity, low cost, and almost endless sodium mineral supply, sodium-ion batteries (SIBs) are predicted to replace LIBs. However, SIBs have a poor energy density and storage capacity, as well as a slow discharging and charging rate. Since the discovery of nanomaterials and nanotechnology, which produce brilliantly functioning electrodes, the development of ion batteries has progressed in recent decades.
Due to its high surface area and promising electrical characteristics, graphene has been widely researched as an anodic material. One-dimensional nanotubes and zero-dimensional fullerenes, in particular, have been used as anodic materials in LIBs and have shown improved electrochemical performance.
When compared to 3D graphite, however, these carbon-based materials only have a short-term performance advantage. It is well known that the morphologies and structures of nanomaterials have a significant impact on their performance. As a result, graphene nanosheets are predicted to boost electrochemical activity. Despite these efforts, there are few publications on boron intercalation within the layers of high-quality graphene nanosheets for enhanced anodic boron-ion batteries (BIBs).
About the Study
In this study, the authors used first-principles computations to study the performance of B3+ on mono-layer (MG), bi-layer (BG), tri-layer (TG), and tetra-layer (TTG) graphene sheets. Using first-principles calculations within the framework of density functional theory (DFT), the high-energy-density anodic component for boron-ion batteries was investigated as an alternative to sodium-ion and lithium-ion batteries. On MG, BF, TG, and TTG graphene sheet electrodes, the electrochemical performance of the boron ion(s) was examined.
The team obtained the most favorable and deeper van der Waals contacts by raising the layers to TTG graphitic carbon B3+@TTG_asym and B3+@TTG_sym. A theoretical study of the anodic performance of graphene nanosheets for boron-ion batteries was presented. The electrochemical performance of single-layer to four-layer graphene sheets were examined.
The researchers paved the road for the successful design of highly efficient energy storage materials. DFT, as implemented in the Gaussian 09 package, was used to accomplish all structural optimizations and electronic property computations. The Perdew–Burke–Ernzerhof (PBE) functional from the generalized gradient approximation (GGA) functional was used to account for the exchange-correlation energy as it provided adequate accuracy, fast computing, and the 6–311 G(d,p) basis set was employed.
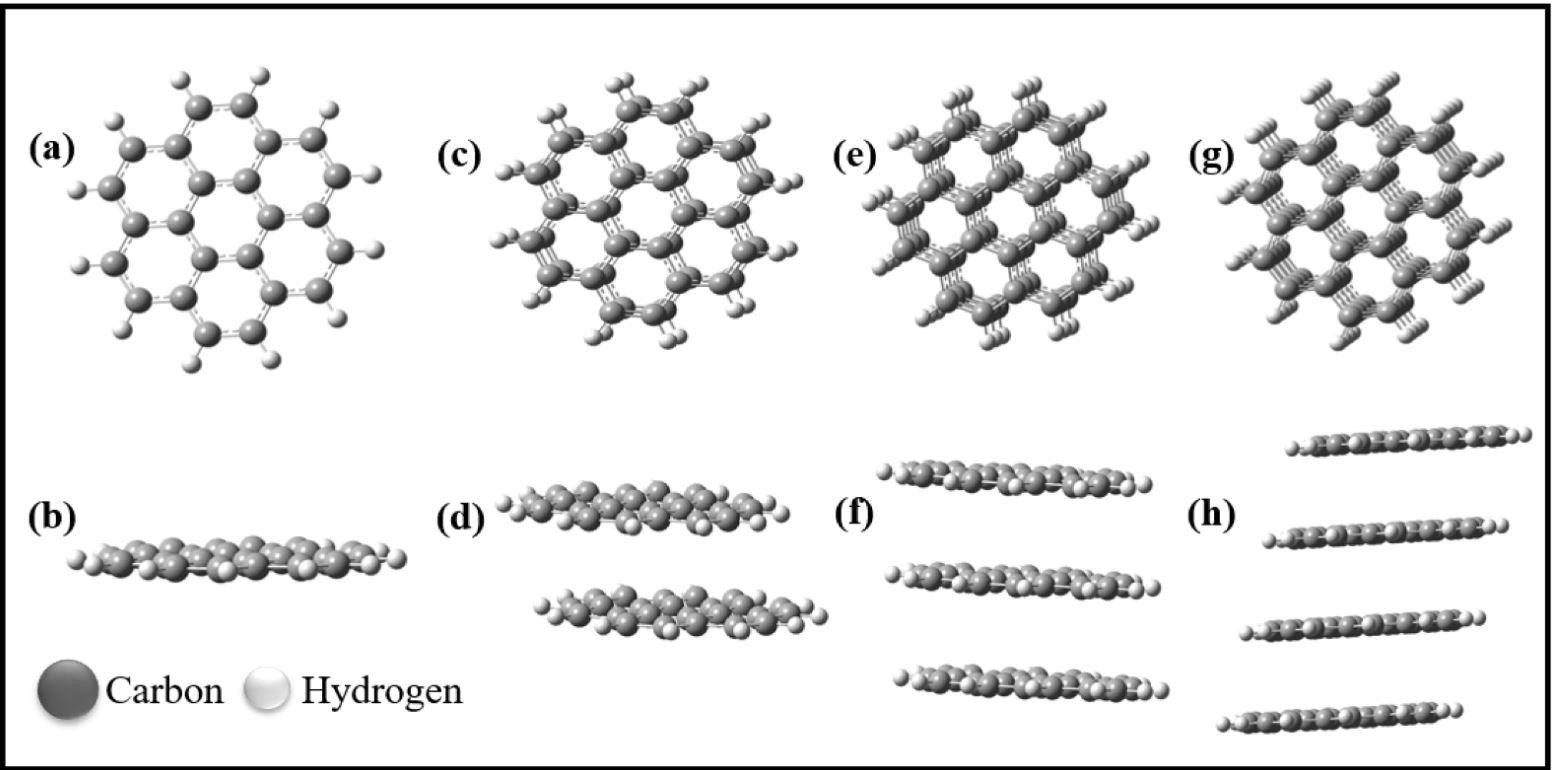
Optimized structures of (a,b) monolayer graphene sheet (MG), (c,d) bilayer graphene sheet (BG), (e,f) trilayer graphene sheet (TG), and (g,h) tetralayer graphene sheet (TTG). (b,d,f,h) Side-view orientation of the graphene sheets. Image Credit: Umar, M et al., Nanomaterials
Using Grimme's D3 method, the influence of dispersion-corrected functionals on the geometry of the layers was also investigated.
Further, the influence of the number of layers and boron ions on the cell voltage was examined. The density of states (DOS) plot was created using the Gauss-Sum software.
Observations
The results showed that adsorption of B3+ caused significant stability of the graphene sheets' HOMO and LUMO frontier orbitals, with energies moved from -5.085 eV to -2.242 eV in B3+@MG and from -20.08 eV to -19.84 eV in 2B3+@TTG. The cell voltages were significantly increased, and B3+/B@TTG had the maximum cell voltage of 16.5 V.
According to the decreased density gradient iso-surface analysis, the most beneficial interactions for the graphene layers were van der Waals, wherein the B3+@TTG asym and B3+@TTG sym configurations displayed the most favorable interactions.
The electrochemical cell voltages were obtained using single-layer (B3+/B@MG) and multilayer (B3+/B@BG, B3+/B@TG, and B3+/B@TTG) graphene sheets were also greatly improved, and B3+/B@MG exhibited the lowest voltage of 13.7 V. On the other hand, a maximum voltage of 16.5 V was found in B3+/B@TTG. The findings also showed that when the number of graphene layers increased, the electrochemical performance of the boron-ion battery's anodic electrode was also enhanced.
Conclusions
In conclusion, this study's results showed that the number of layers or thickness of graphene nanosheets is a useful parameter for the tuning of the anode performance in boron-ion batteries. The authors emphasized that this could point to a new way to improve the graphene anode's performance by incrementing the number of graphene layers.
The theoretical investigations demonstrated the suitability of graphene-based anodic electrodes in boron-ion batteries for large-scale application as a potential energy storage substitute for sodium- and lithium-based ion batteries, which can be beneficial to the design of the material of ion batteries.
The team also observed that increasing the number of graphene layers could be an innovative way to improve graphene anode performance.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.