To address the challenges presented by climate change and human activity, research has focused on the manufacture of environmentally friendly devices for energy harvesting and storage. Devices must meet or exceed the performance of conventional technologies if fossil fuels, one of the main causes of greenhouse gas emissions, are to be phased out in the coming years.
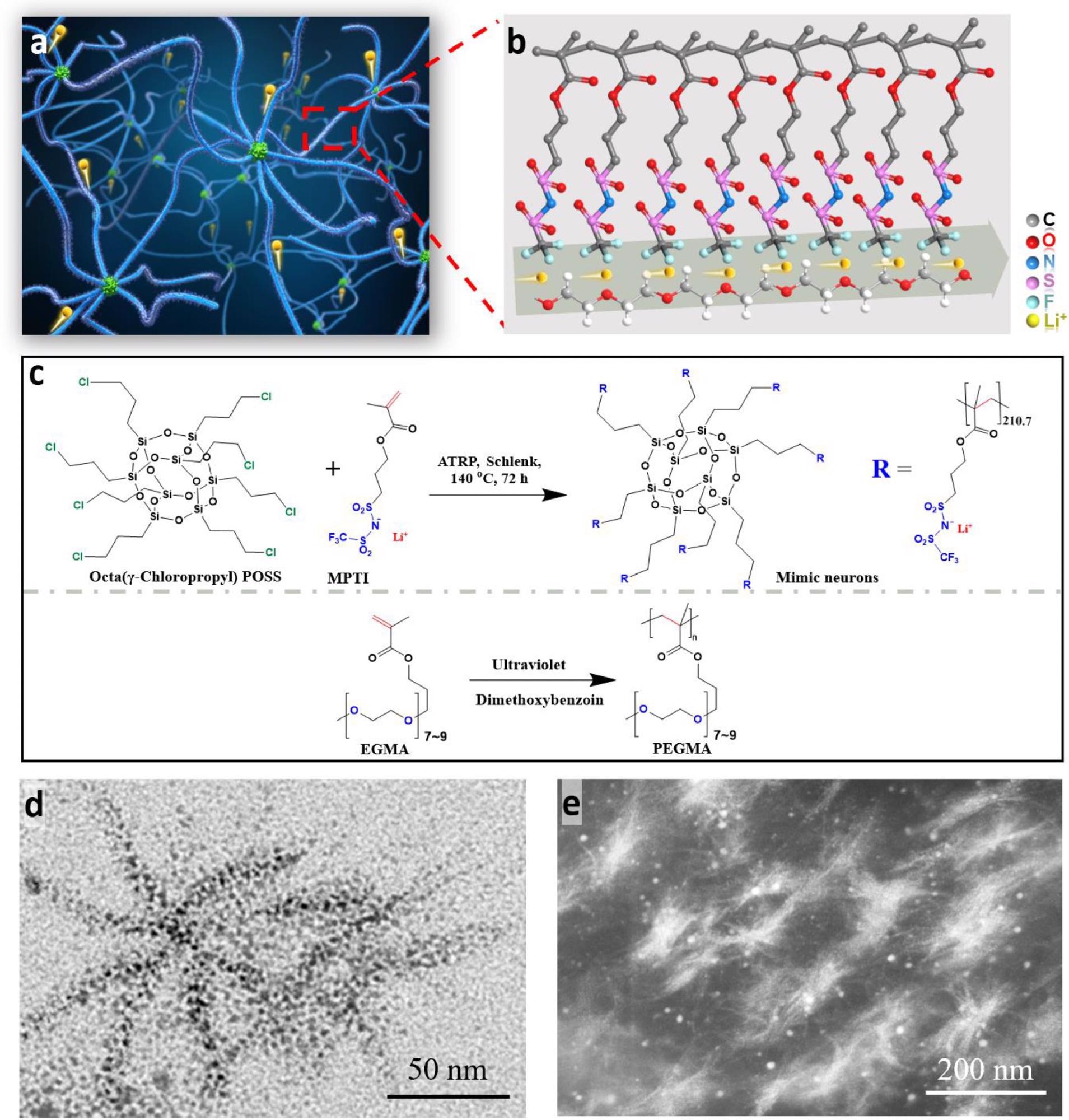
Structure of NN-SPE. (a) and (b) Illustration of the conceptual NN-SPE structure. (c) Illustration and synthetic steps for the preparation of the mimic neurons and mimic neuroglia. (d) HRTEM image of the mimic neuron. The black regions indicated the location of lithium salt groups in the mimic neurons. (e) DF-STEM image of the NN-SPE film. The bright region shows the mimic neuron. Image Credit: Li, Z et al., Energy Storage Materials
Solid-state electrolytes are attracting significant research attention in the field of next-generation energy storage devices. They offer the possibility of developing safer, eco-friendly, and highly efficient energy storage capabilities compared to conventional systems. These electrolytes have been explored for use in fuel cells, lithium batteries, and concentration cells.
In lithium batteries, these electrolytes offer devices significantly enhanced theoretical capacity and can suppress the formation of lithium dendrites, avoiding critical issues such as short-circuiting and improving the safety and lifetime of batteries. Moreover, research in this area opens the possibility of new lithium chemistry-based devices such as lithium-sulfur and lithium-oxygen batteries.
Solid Polymer Electrolytes
Solid polymer electrolytes have become a key area of research in recent years. These electrolytes offer several benefits in terms of cost, stability, processability, and diversity in structure. Additionally, near single-ion lithium conductivity can be achieved with solid polymer electrolytes.
This property is important because it avoids the accumulation of migrated anions at the electrolyte/electrode interface, which aids stable dendrite-free lithium electrodeposition. However, these electrolytes suffer from comparatively low Li-ion conductivity. This hinders their performance and widespread commercial application.
In recent years, research has turned to the development of composite electrolytes which possess hierarchical structures. Studies have reported that incorporating fillers helps to reduce the polymer’s crystallinity. This, in turn, decreases transport resistance and improves the conduction of ions at the interface of polymers and fillers.
Several papers have reported the use of fillers with different structures, such as rods, nanowires, and particles. From the body of research, bi-continuous structures have emerged as potentially advantageous for solid polymer electrode design as interconnected polymer/filler interfaces facilitate rapid ion transport across the entire structure. Anisotropic dendritic nanowires, which have high coordination numbers, easily form fully connected bi-continuous networks.
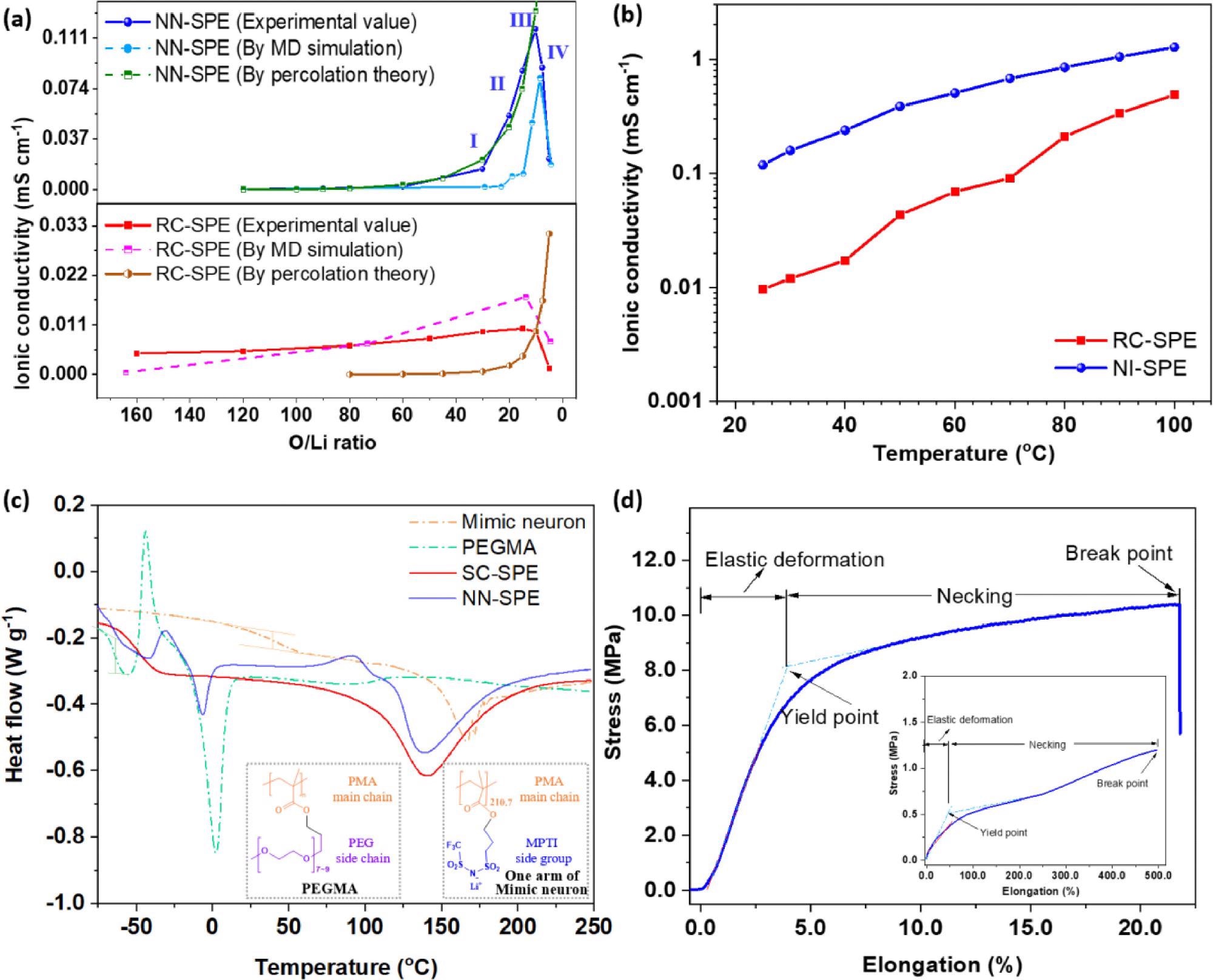
(a) Experimental and the simulated lithium-ion conductivities of NN-SPE and RC-SPE under various O/Li values. The O/Li value was converted from the mass content of the Li-MPTI group (ω, %) using the equation "O". (b) Lithium-ion conductivities of NN-SPE and RC-SPE at the optimum O/Li ratio for NN-SPE (9.52) under different temperatures. (c) DSC curves of Mimic neuron, PEGMA, NN-SPE and RC-SPE; the inserted figure shows the structure of PEGMA and one of the arms of mimic neuron. (d) Mechanical strength curve of NN-SPE. Image Credit: Li, Z et al., Energy Storage Materials
The Study
The team behind the new study has taken inspiration from a common bi-continuous network found in nature: the biological neural network. This novel biomimicking electrode architecture provides faster ion-transport networks, significantly enhancing the speed of Li-ion conductivity in batteries.
The novel electrode structure consists of a core node with eight artificial axions which mimic the natural structure of neural pathways. The mimic axions were polymerized from an Li-MPTI salt. These synthetic neurons were then dispersed into a PEGMA polymer matrix, thus creating the bi-continuous network structure.
Detailed simulations and experimental studies have been conducted in the paper, which reveal that the novel biomimicking electrolyte can easily form a bi-continuous network. The network was formed at a low percolation threshold.
DSC measurements and DF-STEM observations confirmed the electrolyte’s structure. Lithium plating/stripping experiments were performed on the electrolytes, with SEM results indicating a smooth surface structure after cyclical testing, further confirming the suppression of lithium dendrite formation.
Results of the Study
The biomimicking solid polymer electrode architecture displayed superior performance and properties. Li-ion conductivity was improved by orders of magnitude over conventional electrolytes. The electrolyte possesses a high lithium transport number, tensile strength, and Young’s modulus.
The performance of the novel solid polymer electrolyte presented in the paper is higher than currently reported electrolytes. Simulations of molecular dynamics revealed enrichment of lithium ions at the polymer matrix/mimic axion interfaces. Essentially, the polymer matrix mimics the myelin sheath of biological neurons, which facilitates rapid Li-ion transport.
The authors constructed lithium-metal batteries incorporating this novel solid polymer electrolyte. A 1400 h battery test was employed in the research to explore the device’s efficiency and safety. Results demonstrated competitive discharge capacities and lithium dendrite formation suppression, indicating that the use of this novel electrolyte can improve the performance and safe operation of future lithium chemistry-based batteries.
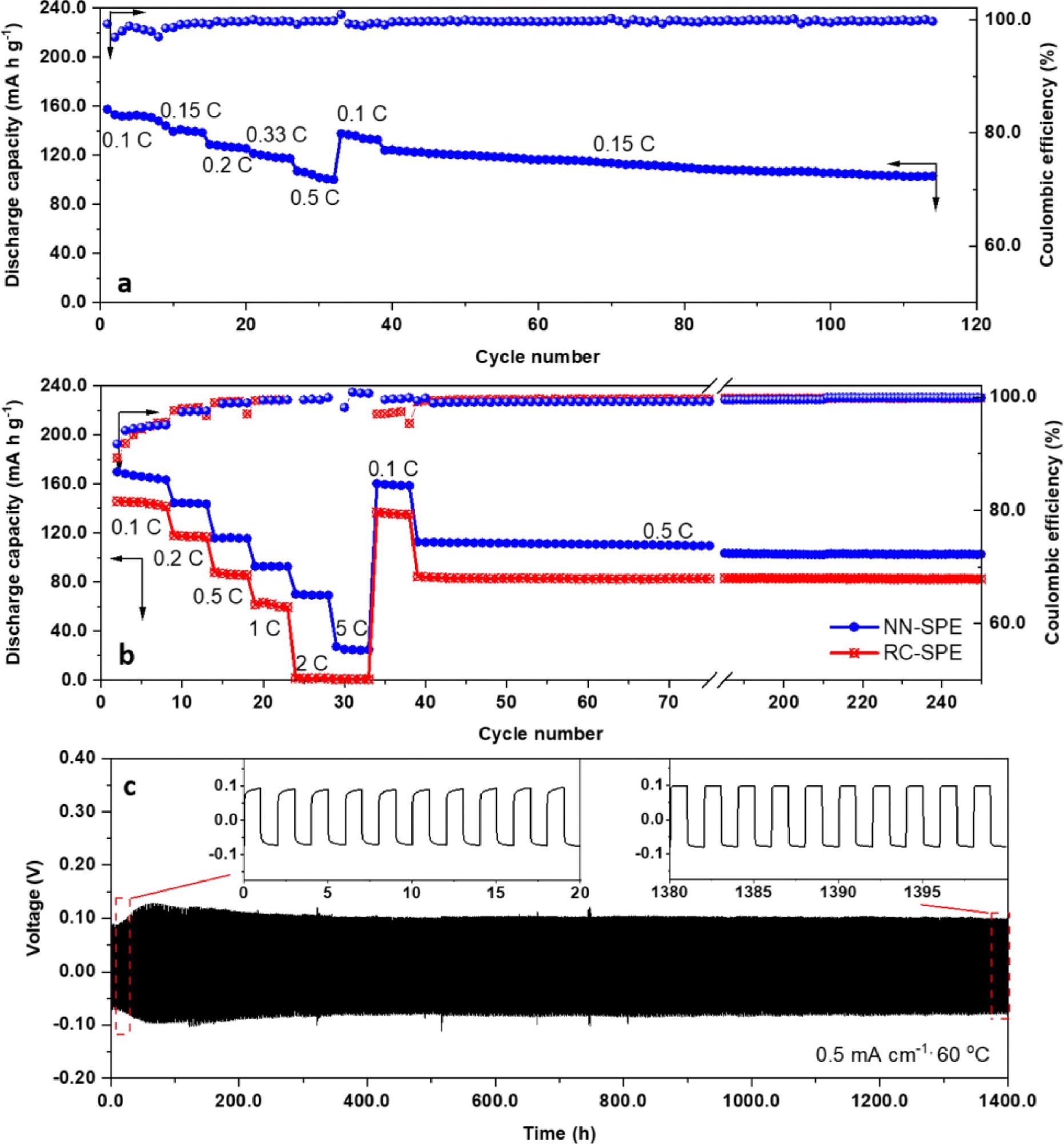
Lithium metal battery performance. (a) Rate and cycling performance of NN-SPE at 30 °C. (b) Rate and cycling performance of NN-SPE at 60 °C. (c) Performance of symmetric lithium cells (NN-SPE) at 0.5 mA cm−1 and 60 °C. Image Credit: Li, Z et al., Energy Storage Materials
In Summary
The authors have introduced a new strategy for developing solid polymer electrolytes for use in Li-ion batteries that significantly improves the performance and safety of conventional electrolytes. By mimicking organic neural networks, the novel electrolyte significantly improves Li-ion conductivity in batteries, which addresses a key challenge in solid polymer electrolyte design. The research provides a novel approach for the design of future Li-ion batteries.
Further Reading
Li, Z et al. (2022) Nerve Network-Inspired Solid Polymer Electrolytes (NN-SPE) for Fast and Single-Ion Lithium Conduction Energy Storage Materials [online, pre-proof] sciencedirect.com. Available at: https://www.sciencedirect.com/science/article/pii/S2405829722002392
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.