A team of Chinese researchers has presented a novel method for efficient solar water splitting. The approach demonstrated in the new paper in ACS Applied Materials involves modifying bismuth vanadate anodes to enhance charge separation. The method provides a simple modification route and a promising design strategy.
Study: Efficient Solar Water Splitting via Enhanced Charge Separation of the BiVO4 Photoanode. Image Credit: Fred Mantel/Shutterstock.com
Photoelectrochemical Water Splitting
The urgent need to phase out greenhouse gas-emitting fossil fuels has facilitated research into alternative fuel production. Hydrogen is an abundant, near-carbon neutral, and renewable resource that can meet the energy demands of a decarbonizing society.
Photoelectrochemical water splitting (PEC) is a green strategy for producing hydrogen from one of the most abundant, cleanest, and renewable sources on earth – water. Photoelectrochemical materials are used to harvest light energy from the sun, and then dissociate water into its constituent parts – hydrogen and oxygen. Hydrogen is then recovered to produce value-added products such as electricity or fuel, and oxygen is released as a clean by-product of the reaction.
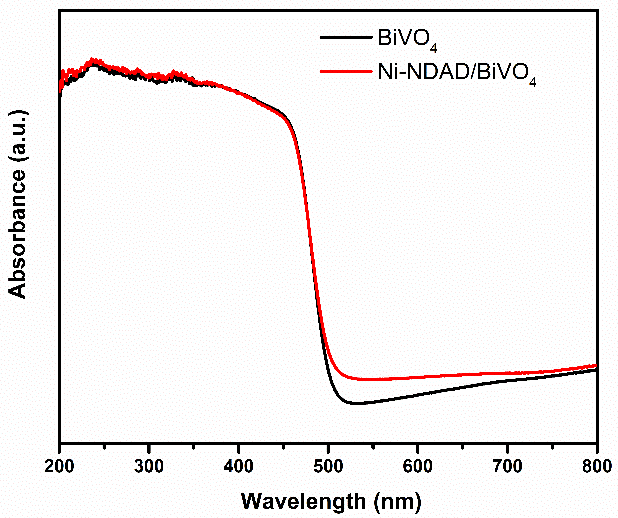
UV–vis diffused reflectance spectra of pure BiVO4 and Ni-NDAD/BiVO4. Image Credit: Wang, L et al., ACS Applied Energy Materials
Photoanodes
In a photoelectrochemical water splitting system, the selection of materials is essential to ensure the efficiency of reactions. Over the years, many materials have been explored for photoanodes, with the earliest reported material being TiO2. Other photocatalysts that have been investigated for use in PEC devices include ZnO, Fe2O3, WO3, and BiVO4 (bismuth vanadate).
Amongst these, monolithic BiVO4 has proven to be an attractive semiconductor material, possessing a narrow band gap and appropriate band edge location for reactions. However, this material possesses limitations, mainly its unfavorable recombination rate of electron holes and slow water oxidation kinetics. These drawbacks cause the practical efficiency of reactions to be lower than their theoretical efficiency.
Using composite materials is a strategy to overcome the drawbacks of this photocatalytic material. Research has developed composites of functional materials, including CoOOH/BiVO4 and Co3O4/BiVO4. Incorporating functional materials into composites improves the anode’s charge separation and kinetics, promoting more efficient water splitting.
In recent years, researchers have investigated the use of metal complexes such as cobalt, nickel, and iron for PEC reactions. These materials have attracted attention because of their enhanced performance as catalysts, rich structures, and diversified functionalities.
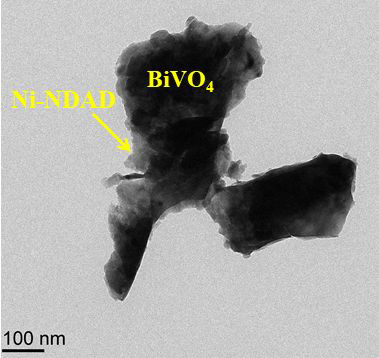
The TEM image of Ni-NDAD/BiVO4. Image Credit: Wang, L et al., ACS Applied Energy Materials
The Research
Whilst there has been a growing body of research on the incorporation of metal complexes into BiVO4 photoanodes to fabricate composite materials with the effect of improving the efficiency of water splitting reactions, there has been little research on incorporating Ni-NDAD layers into BiVO4 composites. The team used the long-term hydrothermal method to produce the BiVO4/Ni-DAD composite photoanode.
The prepared material displays a superior photocurrent density compared to pure BiVO4 in an electrolyte of potassium borate. The figures obtained were more than three times those displayed by pure BiVO4. Analysis revealed that the presence of elevated amounts of active sites and the ultrathin structure of Ni-NDAD contributed to the enhanced charge separation in the composite BiVO4/Ni-NDAD material.
Transmission electron microscopy revealed the presence of tight interactions between the BiVO4 and Ni-NDAD layers, which promoted efficient charge transfer. The authors have noted that this could be beneficial for the design of highly efficient PEC systems. Ni-NDAD plays a key role in the conversion of light energy to electrical energy.
Another key finding in the research was the enhanced catalytic activity of the composite material. The PEC catalytic activity was evaluated on chlortetracycline, an antibiotic, in persulfate. The degradation activity of this antibiotic was promoted by the novel photocatalyst presented in the research and reached 89.19% within thirty minutes. This activity is beneficial as there is growing attention on the release of antibiotics into the environment and the rise of drug-resistant bacteria.
The authors investigated the stability of the proposed material, which is crucial for practical applications. They performed three repeated experiments on chlortetracycline degradation, demonstrating no notable reduction in photocatalytic activity. SEM images and XRD patterns displayed almost no change, indicating the material’s stability.

SEM images of Ni-NDAD/BiVO4 photoanodes after PEC degradation of CTC test. Image Credit: Wang, L et al., ACS Applied Energy Materials
In Summary
The research has demonstrated a promising photoanode for PEC water splitting applications that combines BiVO4 and Ni-NDAD. The prepared material presented in the research displays significantly enhanced charge separation and can deliver a high photocurrent density compared to pure BiVO4 photoanodes, improving upon current PEC water-splitting technologies.
Photoelectrochemical water splitting has the potential to provide a clean and sustainable route to green hydrogen production, helping to achieve net-zero global emissions targets by 2050. Whilst there are still significant challenges in the area, this research paper presents an innovative, low-cost, and highly efficient approach to improving PEC water splitting technologies.
Further Reading
Wang, L et al. (2022) Efficient Solar Water Splitting via Enhanced Charge Separation of the BiVO4 Photoanode ACS Applied Energy Materials [online] pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsaem.2c00779
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.