Ammonia, a nitrogen and hydrogen (NH3) compound known best for its use as a household cleaner, can be employed as a vehicle alternative fuel or as a form of energy storage.
 The assembled Zn–NO battery shows superior performance for ammonia production. Image Credit: Nano Research Energy, Tsinghua University Press.
The assembled Zn–NO battery shows superior performance for ammonia production. Image Credit: Nano Research Energy, Tsinghua University Press.
Though some more recent attempts to create ammonia from a NO reduction process have poor ammonia yields, traditional techniques of manufacturing ammonia from nitrogen oxide (NO) on a big scale are not energy efficient. Now, scientists from Tsinghua University Press in China have devised a method for generating ammonia through an electrocatalytic NO reduction reaction that is both energy- and environmentally friendly.
On July 7th, 2022, the research was published in the journal Nano Research Energy.
The industrial-level NH3 production is still heavily relying on the Haber–Bosch process, requiring drastic reaction conditions due to the sluggish kinetics, and the energy input aspect of the process inevitably results in a large amount of greenhouse gas emissions.
Xijun Liu, Study Corresponding Author, School of Resource, Environments and Materials, Guangxi University
His mention of the Haber-Bosch process pertains to one of the first and most popular NH3 production technologies since its invention in the early 1900s.
Due to these difficulties, earlier research has focused on electrocatalytic ammonia production in water, which uses single-atom catalysts, carbon-based materials, and noble metal-based materials to produce ammonia with zero carbon emissions. However, the researchers found that the yield rate was significantly lower than the US Department of Energy target.
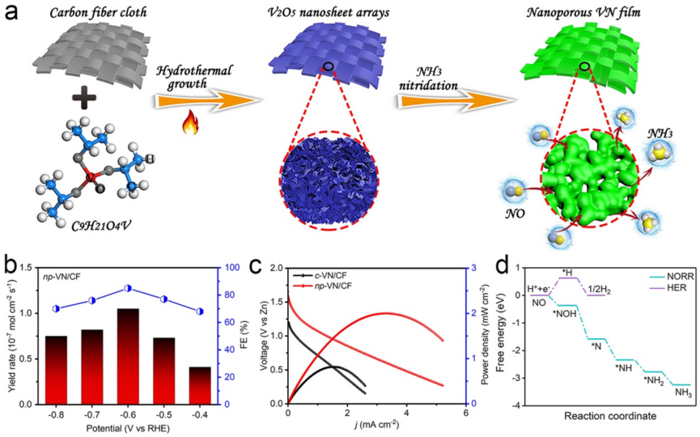
The scientists created a nanoporous vanadium nitride (VN) film supported on carbon fiber cloth (written as np-VN/CF) to study the NO reaction reduction (NORR) to ammonia. They used a zinc–nitrogen oxide battery to test the unique technique, which involves utilizing already-existing components of VN film in a novel manner and for a novel purpose.
This designed catalyst shows a high power density and a high corresponding ammonia yield rate when used as the cathode in a home-assembled Zn–NO battery. We also found that the faraday efficiency ¾ or how well the electrons, or charge, are transferred in an electrochemical transformation ¾ was improved. The achieved NORR performance metrics are comparable to the recently reported best results.
Xijun Liu, Study Corresponding Author, School of Resource, Environments and Materials, Guangxi University
The fact that some nitrogen was “doped” into carbon fiber fabric, increasing its likelihood of being highly conductive and favoring the charge transfer, can be partly blamed for the high faraday efficiency.
Scientists bubbled NO feeding gas into the cathode chamber of the battery, which is the negatively charged area of a battery and is isolated from the positive charge by a bipolar membrane, to test the procedure with the battery.
The researchers found that the Zn–NO battery performed better than data that had previously been published. The results of the studies were tested in a three-electrode system, and the data were contrasted with the NORR activity of a commercial VN powder cast onto a carbon fiber fabric, as opposed to film. In every measurement, the VN film version performed better than the powder version that had been utilized in earlier studies.
To provide a comprehensive understanding of NORR, the investigators also ran calculations using the density functional theory.
Our work shows the potential application of nanoporous materials for high-performance electrochemical NH3 production.
Xijun Liu, Study Corresponding Author, School of Resource, Environments and Materials, Guangxi University
The research’s next steps would involve making an effort to scale the proof-of-concept presented in this experiment.
Liu says, “This work further confirms that the electroreduction of NO is a promising strategy for ambient ammonia synthesis that should be continuously developed.”
This study was supported by funds from the Tianjin Science Fund for Distinguished Young Scholars and the Natural Science Foundation of China.
Defeng Qi of the School of Resource, Environments and Materials at Guangxi University and of the School of Materials Science and Engineering at Tianjin University of Technology, Tianjin China; Fnag Lv, Mengmeng Jin and Jun Luo of the School of Materials Science and Engineering at Tianjin University of Technology; Tianran Wei and Dui Ma of the School of Resource, Environments and Materials at Guangxi University contributed to the study.
The other authors are Ge Meng of the College of Chemistry and Materials Engineering at Wenzhou University in Wenzhou, China; Shusheng Zhang of the College of Chemistry at Zhengzhou University in Zhengzhou, China; Qian Liu of Chengdu University in Chengdu, China; Wenxian Liu of the College of Materials Science and Engineering; and Mohamed S. Hamdy of the Department of Chemistry, College of Science at King Khalid University in Abha, Saudi Arabia.
Journal Reference:
Qi, D., et al. (2022) High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Research Energy. doi.org/10.26599/NRE.2022.9120022.