The production of nitrogen fertilizers requires ammonia. It has been a long-term objective to find alternate materials and methods for effective N2 reduction to ammonia under benign settings due to the significant energy consumption of industrial ammonia synthesis.
 Transition-metal-free dinitrogen fixation mediated by barium hydride. Image Credit: Yeqin Guan
Transition-metal-free dinitrogen fixation mediated by barium hydride. Image Credit: Yeqin Guan
Prof. Tejs Vegge of the Technical University of Denmark and Prof. Ping Chen of the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently worked together to synthesize ammonia through a chemical looping process that was mediated by a transition-metal-free barium hydride (BaH2) and reveal its mechanism.
On August 2nd, 2022, this study was released in the Angewandte Chemie International Edition.
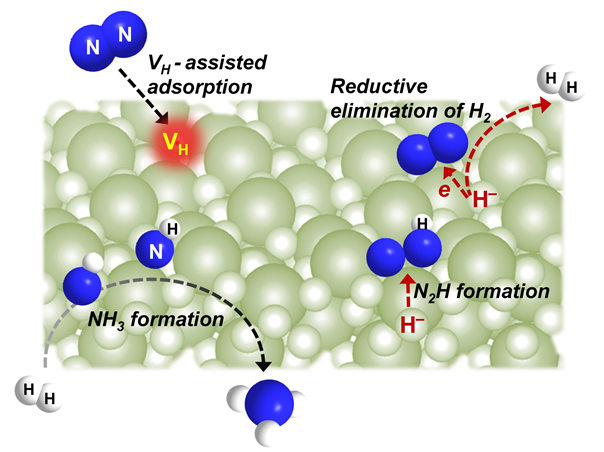
When N2 is fixed by alkali or alkaline earth metal hydrides, matching metal imides and H2 are produced. The metal imides are then hydrogenated to produce metal hydrides and NH3. However, it is still unknown how alkali hydrides cause N2 activation, H2 release, and NH3 production.
The researchers claimed that BaH2’s participation in the N2 fixation process, which is mediated by hydrogen vacancies, was crucial.
Multiple coordinatively unsaturated Ba sites were formed due to the development of hydrogen vacancies, and these sites were in charge of the adsorption and activation of N2. As an electron donor, the hydridic hydrogen aided in the activation of N2 and the contemporaneous release of H2.
They discovered that the method functioned similarly to nitrogenase's FeMo cofactor and molecular hydrido complexes. Gaseous H2 and hydridic hydrogen both contributed to the production of NH3.
This is a helpful model for understanding the activation and hydrogenation of N2 to NH3 mediated by alkali and alkaline earth metal hydrides, which is promising in future technologies for nitrogen fixation using transition-metal-free materials.
Prof. Ping Chen, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
The National Natural Science Foundation of China and the CAS Youth Innovation Promotion Association provided funding for this research.
Journal Reference:
Guan, Y., et al. (2022) Transition-Metal-Free Barium Hydride Mediates Dinitrogen Fixation and Ammonia Synthesis. Angewandte Chemie International Edition. doi:10.1002/anie.202205805.