Reviewed by Mila PereraOct 25 2022
Due to a new technique developed by scientists at Tokyo Tech based on a 13C-13C abundance analysis, the secret behind the origin of hydrocarbons discovered in extraterrestrial environments might be finally uncovered.
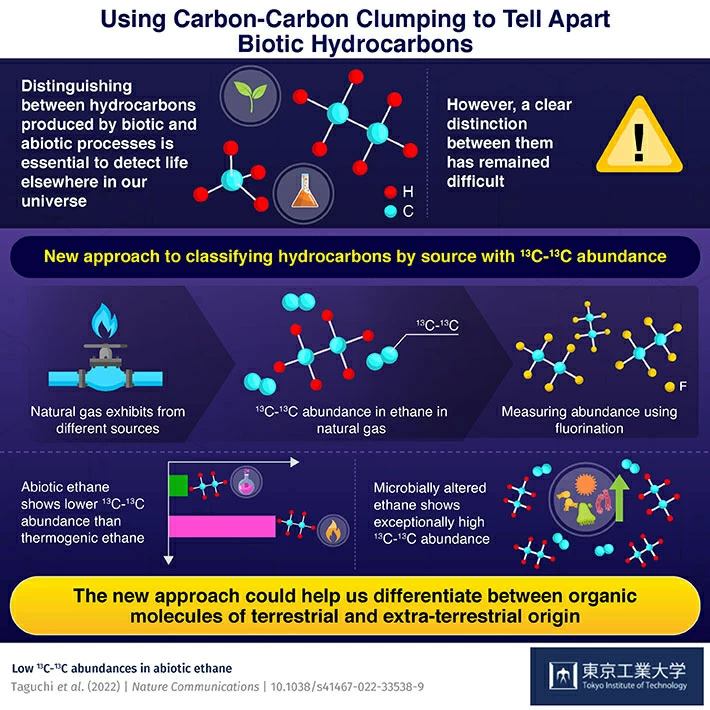
Image Credit: Tokyo Institute of Technology
By measuring the abundance of the clumped 13C-13C isotope in the hydrocarbons, it can be deduced whether a hydrocarbon was produced through biological processes. This could pave the way for distinguishing such hydrocarbons from abiotic ones, thereby helping the search for extraterrestrial life.
A significant signature of life is the existence of organic molecules that might originate from biological processes. The most general organic molecule discovered in all life forms is hydrocarbons.
However, they do not need to be of biotic origin, that is, produced from the thermal decomposition of microbes or sedimentary organic matter. Hence, while hydrocarbons have been discovered in various places outside of Earth, they are not necessarily suggestive of extraterrestrial life.
Such hydrocarbons could well have been shaped by abiotic, or non-biological processes. Therefore, determining if a hydrocarbon is of biotic or abiotic origin is critical to deduce the existence of life. Unfortunately, this has proved to be a tremendously challenging task.
A research group headed by Professor Yuichiro Ueno from the Tokyo Institute of Technology (Tokyo Tech) has risen to this challenge.
While methods to distinguish the source of the hydrocarbon, such as compound-specific isotope analysis, are available, they require a whole set of molecules, all of which are not always available to sample. In contrast, our method allows us to use the information contained in the molecule to find the source of its origin.
Yuichiro Ueno, Professor, Tokyo Institute of Technology
The group accounts for a robust, novel method to differentiate between the sources of hydrocarbons by studying the comparative abundance of an isotope of carbon, the so-called 13C-13C, in organic molecules.
The study has been reported in Nature Communications.
To leverage this data, the team considered the comparative abundance of various isotopes of carbon in ethane. They compared the abundance of ethane molecules consisting of both 12C atoms, having both 13C atoms, and one 12C and one 13C atom.
Based on this, the team assessed the abundance of 13C-13C in the ethane molecules present in the sample. Then, they compared the value of this abundance of 13C-13C in natural gas ethane with that synthesized in the lab.
They discovered that 13C-13C abundance in natural gas ethane, produced through the thermal decomposition of organic matter, was comparatively greater than expected based on the natural abundance of 13C.
According to the group, this is due to the carbon bonding in the organic molecules that produce natural gas. This contrasted with the abiotically produced ethane, which demonstrated considerably low 13C-13C abundance. Moreover, they noted that microbially-produced ethane consisted of even higher 13C-13C abundance than thermogenic ethane.
This new approach can help us identify the origin of organic molecules, both on earth and in extraterrestrial environments. It can easily differentiate between thermogenic, abiotic, and microbially produced hydrocarbons.
Yuichiro Ueno, Professor, Tokyo Institute of Technology
Ueno added, “While more interlaboratory work needs to be done for further calibration of the method, we believe it can potentially help detect the signatures of life elsewhere the universe.”
Journal Reference:
Taguchi, K., et al. (2022) Low 13C-13C abundances in abiotic ethane. Nature Communications. doi.org/10.1038/s41467-022-33538-9.