Reviewed by Emily Henderson, B.Sc.Nov 2 2022
Sodium secondary batteries exhibit an identical functioning principle to lithium secondary batteries, but they come at a lower price and are expected to function as a potential complement to lithium secondary batteries. Solid-state batteries provide high-energy and high-safety density characteristics and are regarded to equip remarkable prospects.
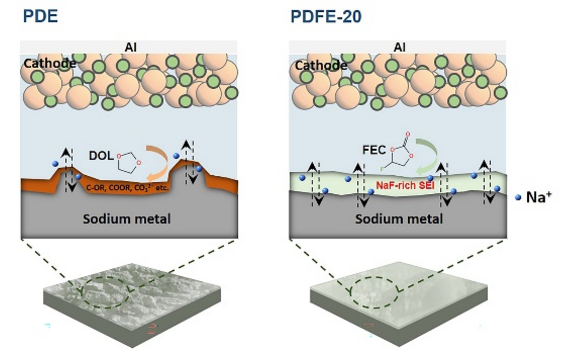 A poly(1,3-dioxolane)-based quasi-solid electrolyte for sodium metal batteries were prepared by in situ polymerization, and the crucial roles of FEC toward forming a stable solid electrolyte interphase (SEI) and preventing interfacial side reactions were demonstrated. Image credits: Journal of Energy Chemistry
A poly(1,3-dioxolane)-based quasi-solid electrolyte for sodium metal batteries were prepared by in situ polymerization, and the crucial roles of FEC toward forming a stable solid electrolyte interphase (SEI) and preventing interfacial side reactions were demonstrated. Image credits: Journal of Energy Chemistry
All-solid-state batteries, however, have the drawback of low ionic conductivity and poor physical solid-solid interfacial contact, which limit their performance and hamper their short-term commercial applicability. Recently, the in situ polymerization usage in preparing solid/quasi-solid electrolytes has been well-studied and has allowed close interfacial contact between the electrodes and the electrolyte.
Several in situ polymerization systems have embraced a high-temperature free-radical polymerization strategy; retaining high reaction temperatures for an extended period is unfavorable to the battery's performance. At room temperature, 1,3-dioxolane (DOL) can be polymerized via cationic ring-opening reactions to gain poly(1,3-dioxolane) (PDOL), and it has high application and research value.
Recently, DOL has been productively employed as an in situ polymerization monomer in lithium metal/lithium-sulfur batteries, but there is little research on the application of PDOL in sodium metal batteries (SMBs). Additionally, even though in situ polymerization strategies could highly impact the physical contact between the electrodes and electrolytes, it is still difficult to sustain close interfacial contact in battery cycling when the high reactivity of the Na metal anode is considered.
Lately, Hongfa Xiang’s team from the Hefei University of Technology and David Mitlin’s team from the University of Texas in Austin reported a manuscript in the Journal of Energy Chemistry.
This research is based on the interfacial protection strategy. This study introduced fluoroethylene carbonate (FEC) into the PDOL system. A PDOL-FEC quasi-solid electrolyte was made by the in situ polymerization technique and efficiently applied to SMBs.
DOL reacted continuously with the highly chemically active Na metal anode, and the reaction products’ accumulation resulted in high interfacial resistance, which worsened the batteries’ performance. Upon the FEC introduction, it was reduced at the Na metal anode side before DOL addition, creating a NaF-rich passivation layer and hindering extra side reactions between DOL and the Na metal, thereby successfully enhancing the complete performance of the batteries.
Journal Reference:
Ma. J. et al. (2022) Stable sodium anodes for sodium metal batteries (SMBs) enabled by in-situ formed quasi solid-state polymer electrolyte. Journal of Energy Chemistry. https://doi.org/10.1016/j.jechem.2022.09.040.