PEs play a critical role in the development of energy-dense batteries and flexible energy devices owing to their mechanical and chemical reliability. However, the ionic conductivity value of PEs with suitable mechanical properties is significantly lower at room temperature compared to the conductivity value of 10−4 S cm−1 required for practical applications, which hinders their large-scale adoption.
The mechanical properties and ion conductivity of PEs are inversely related, which significantly increases the challenges of properly balancing both properties for practical applications. Polyacrylates (PAs) have gained significant attention as suitable PE hosts owing to their mechanical durability, chemical tunability, electrode compatibility, and ester polarity.
PA is used extensively in emerging composites, dry solids, and plasticized gels, which makes PA-based PEs a suitable option for investigating the trade-off between mechanical strength and ion conduction in PEs.
In this paper, the authors reviewed the major strategies used to balance and reconcile the mutually exclusive mechanical and ion transport properties in PA-based PEs to identify the next-generation PE design guidelines.
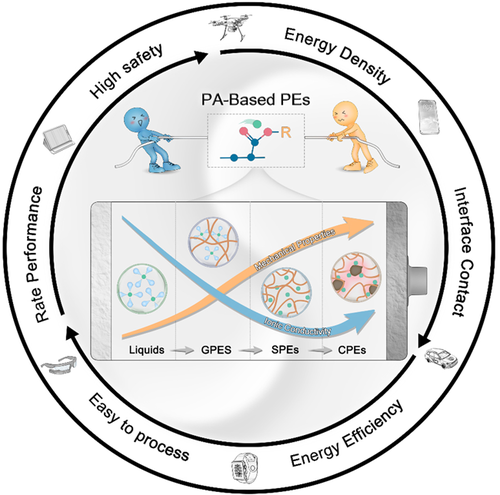
Trade-off between ionic conductivity and mechanical properties in PA-based PEs. PA, polyacrylate; PE, polymer electrolyte. Image Credit: Du, J et al., Carbon Energy
Alkyl‐PA‐based Gel Polymer Electrolytes (GPEs)
Polymethyl methacrylate (PMMA) is used extensively as GPE skeletons owing to its exceptional lithium metal compatibility, ester polarity, high strength, and high glass-transition temperature (Tg). The good ester-solvent affinity of PMMA prevents adverse interfacial reactions and leakage. Additionally, ester groups attached to alkyl-PMMA backbones can facilitate salt dissociation.
Poly ethyl acrylate (PEA)-based GPEs plasticized using one M lithium hexafluorophosphate in ethylene carbonate-dimethyl carbonate (EC‐DMC) demonstrated the highest ionic conductivity of 6.30 mS cm−1 among all alkyl-PA-based PEs. Moreover, the PEA-based GPEs displayed both rigidity and elasticity.
Long alkyls in PAs improve the flexibility, ductility, ion conduction, and compatibility and adhesion of GPEs with lithium anode, while the methyl group tethered to main chains increases the GPE brittleness. Alkyls containing strongly polar fluorine can promote thermal and mechanical stability and facilitate salt dissociation.
The pore density and structure of polymer films can be controlled in a cost-efficient manner using in situ acrylate monomer polymerization to improve the mechanical properties. For instance, in situ polymerized poly(butyl acrylate) (PBA)‐based GPE with 80 wt.% liquid content displayed excellent elasticity with over 600% strain at the break.
Plasticizers for alkyl-PA-based GPEs are selected depending on their chemical characteristics, including donor number and dielectric constant and ability to interact with polymer hosts.
For instance, the polarity mismatch between EC/propylene carbonate (PC) electrolytes and butyl groups can result in phase separation, which restricts bulk ion mobility and free volume in GPE films.
The mechanical strength of alkyl‐PA‐GPEs must be improved as their integrity is significantly affected after liquid absorption. Other polymer phases and monomers can be used to blend and copolymerize with alkyl PAs, respectively, to improve mechanical strength.
For instance, the incorporation of polyacrylonitrile (PAN) into PMMA‐based GPEs can improve mechanical properties, salt solubility, processability, flame retardancy, oxidation stability, and thermal stability.
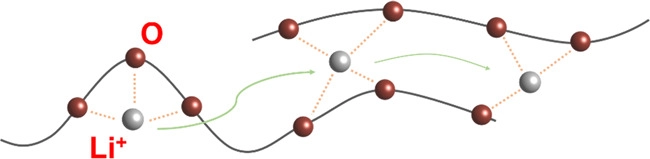
Ion transport mechanism in PEO. PEO, polyethylene oxide. Image Credit: Du, J et al., Carbon Energy
Ethylene Oxide (EO)‐PA‐based GPEs
Polar and flexible EO branches in EO‐PAs, such as poly (poly (ethylene glycol) methacrylate) P(PEGMA), can preserve plasticizers within the polymer hosts, which prevents undesirable electrode-electrolyte mass transfer. A poly(n-butyl methacrylate) (PBMA)-PEGMA copolymer can retain 400 wt.% solvents while maintaining good mechanical stability.
However, the soft comb-like structures EO‐PAs make them dimensionally unstable. Chemical crosslinkers, such as ternary B‐centered PEGMA (BC) and bisphenol A ethoxylated dimethacrylate (BEMA), can be used to improve the modulus of EO-PAs. Additionally, the mechanical properties of EO-PA-GPEs can also be improved significantly by constructing semi‐interpenetrating networks (SIPNs) or interpenetrating networks (IPNs).
The solvent and ion mobility gradually reduce in EO- or alkyl-PA-GPEs due to the sluggish segmental dynamics of EO-PAs/alkyl-PAs, which necessitates the use of a large amount of liquid plasticizers that affect the polymer skeleton dimensional stability. Thus, ensuring mechanical stability is crucial in GPEs.
EO‐PA-based Solid-state Polymer Electrolytes (SPEs)
The lithium-ion transport behavior in EO‐PA SPEs is directly correlated to the EO side chain dynamics. Although oligomeric P(PEGMA) possesses a good conductivity of 10−5 S cm−1, it cannot provide the required dimensional stability, which necessitates the use of other strategies, such as the construction of specialized polymer architectures or coupling with other monomers.
For instance, the cross-linking of a copolymer of PEGMA, (2‐(3‐(6‐methyl‐4‐oxo‐1,4‐dihydropyrimidin‐2‐yl)ureido)ethyl methacrylate), and hexa(4‐ethyl acrylate phenoxy) significantly improved the mechanical strength. However, the degree of cross-linking must be modest to allow unfettered lithium ion conduction and short-range motility.
Excessive cross-linking can be prevented by constructing SIPN that can couple non‐cross‐linked ion‐conductive polymer phases and cross-linked networks to balance mechanical properties and ionic conductivity.
Although simple copolymerization methods can improve the mechanical strength of PEGMA SPE, they also lead to loss of ion transport. Block copolymers can be used to fabricate micro(nano)phase‐separated heterogeneous SPEs containing EO‐rich domains with lithium conduction pathways and a rigid-supporting phase.
Microphase‐separated PEGMA‐based SPEs demonstrate exceptional mechanical properties and a higher ionic conductivity compared to uniform SPEs. Moreover, the properties of nanostructured block copolymer electrolytes can be optimized on demand by adjusting the polymer structure and block chemistry. For instance, the rigid polystyrene (PSt) block can improve the mechanical strength of the viscous P(PEGMA) significantly.
Hyperbranched polymers possess exceptional structural properties, such as tunable chain packing, low crystallinity, controllable flexibility, and heat tolerance due to their rich terminal groups and irregular branches. The functionalized terminal groups can enhance the SPE mechanical properties through physical entanglement without adversely affecting the conductivity.
The short‐range disordered and long‐range ordered plastic crystals can provide a high-entropy medium for lithium-ion migration. For instance, the introduction of succinonitrile (SN) into SPEs improved the conductivity to 10−4 S cm−1. However, salt‐coordinated SN coupled with weakened polymer entanglement can adversely affect the mechanical properties. Cyano groups can be incorporated into polymer side chains to maintain dimensional stability while using SN plasticizers.
Exceptionally high ionic conductivity values near unity can be achieved using single‐ion conductive SPEs. For instance, the conductivity of single‐ion SPE with the pendent trifluorobutane sulfonate moiety was considerably higher compared to nonfluorobutane sulfonate owing to improved charge delocalization.
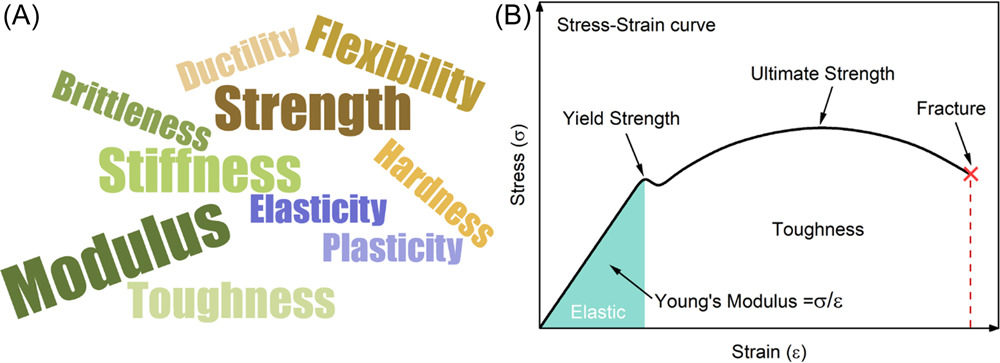
The (A) descriptions and (B) quantifications of mechanical properties. Image Credit: Du, J et al., Carbon Energy
Inorganic Additives for PA-based Composite Polymer Electrolytes (CPEs)
Inorganic components, such as zirconium dioxide, tin oxide, and titanium dioxide, can be chemically bonded with or physically injected into PEs to synthesize hybrid inorganic-organic PEs (HPEs) or CPEs with balanced mechanical performance and ion transport.
Several studies have demonstrated that these inorganic phases can positively impact ion conduction, interface behavior, thermodynamic stability, and lithium-ion dendrite suppression.
For instance, the incorporation of two wt.% silicon in PMMA/poly(vinylidene fluoride-hexafluoropropylene) (P(VDF-HFP))/thermoplastic polyurethane (TPU) plasticized using one M lithium perchlorate in EC/PC increased the conductivity to 8.5 mS cm-1 with 10.8 MPa compressive stress and 86.4% a tensile strain at the break.
Solid inorganic electrolytes (SIEs) can be coupled with SPEs to realize superior ion ‐conducting capability with flexibility, processability, and interface friendliness.
Conclusion
Most PA-based PEs, specifically GPEs, can be tuned based on application demands. However, the limitations associated with the mechanical performance of GPEs must be addressed to use them for practical applications.
Specifically, regulating the optimal plasticizer amount, using non-flammable liquids, and understanding the interaction among salts, polymers, and liquids at the molecular level are necessary.
Additionally, the conductivity of SPEs must be improved to make them technically feasible. The coupling of flexible EO‐PAs with stiff SIEs can potentially balance the mechanical performance and ionic conductivity.
In the future, machine learning and molecular dynamics simulation can be used to accelerate identifying, screening, and predicting desirable material structures and compositions to develop PEs for next-generation energy technologies.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.