Image Credit: Osaka Metropolitan University.
If one has ever noticed water freeze to ice, they have witnessed what physicists call the so-called “phase transition.” Researchers from Osaka Metropolitan University (OMU) have found an unparalleled phase transition at the time when crystals obtain amorphous characteristics while their crystalline properties have been maintained.
Their study outcomes include developing hybrid materials for usage in rough surroundings like outer space. The study has been published in the journal Physical Review B.
A normal phase transition displayed by crystalline solids includes an alteration in the crystal structure. Generally, such structural phase transitions happen at finite temperatures. But regulating the crystal's chemical composition can potentially decrease the transition temperature to absolute zero (−273 °C). The transition point at absolute zero is known as the structural quantum critical point.
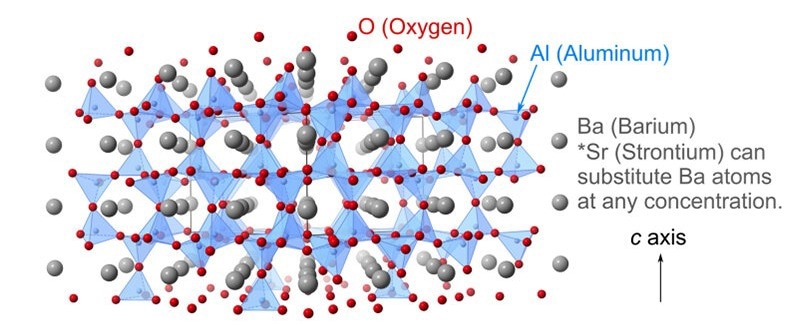
As far as the dielectric compound Ba1-xSrxAl2O4 is concerned, the structural phase transition has been pushed by an acoustic soft mode, the atomic vibration pattern of which is the same as that of sound waves. The compound consists of an AlO4 tetrahedral network and Ba or Sr atoms.
The research group headed by Associate Professor Yui Ishii from the Graduate School of Engineering at Osaka Metropolitan University has found that a highly disordered atomic arrangement has been developed in the AlO4 network at chemical compositions next to the structural quantum critical point. This leads to both characteristics of amorphous and crystalline materials.
Ba1-xSrxAl2O4 is known as a crystalline solid. But the scientists discovered that at greater Sr concentrations compared to the structural quantum critical point, Ba1-xSrxAl2O4 displays the thermal characteristic of amorphous materials, which is the low thermal conductivity relevant to that of glass materials (for example, silica glass).
The researchers noted that a part of the atomic structure tends to lose its periodicity due to the incoherently stopped acoustic soft mode. Consequently, a combination of a periodic Ba and a glassy Al-O network arrangement has been identified.
The research group was the first to discover the hybrid state, and it could be made just by blending raw materials evenly and then heating them.
In principle, the phenomenon revealed in this research can occur in materials exhibiting acoustic soft modes. Applying this technique to various materials will possibly help us create hybrid materials that combine the physical properties of crystals, such as optical properties and electrical conductivity, with the low thermal conductivity of amorphous materials.
Yui Ishii, Professor, Graduate School of Engineering, Osaka Metropolitan University
Ishii added, “In addition, the high heat resistance of crystals can be utilized to develop insulation materials that can be used in harsh environments, such as outer space.”
Journal Reference:
Ishii, Y., et al. (2022) Partial breakdown of translation symmetry at a structural quantum critical point associated with a ferroelectric soft mode. Physical Review B. doi.org/10.1103/PhysRevB.106.134111.