Scientists at Dongguk University investigated a synthetic method to develop new metal oxyhydroxides electrocatalysts for efficient water electrolysis. Their findings were published in a study, which provides a detailed insight into the process demonstrating the long-term stability and performance of the synthesized catalysts. These can be used to obtain hydrogen (H2) and manufacture hydrogen fuel cells as an alternative to fossil fuels.
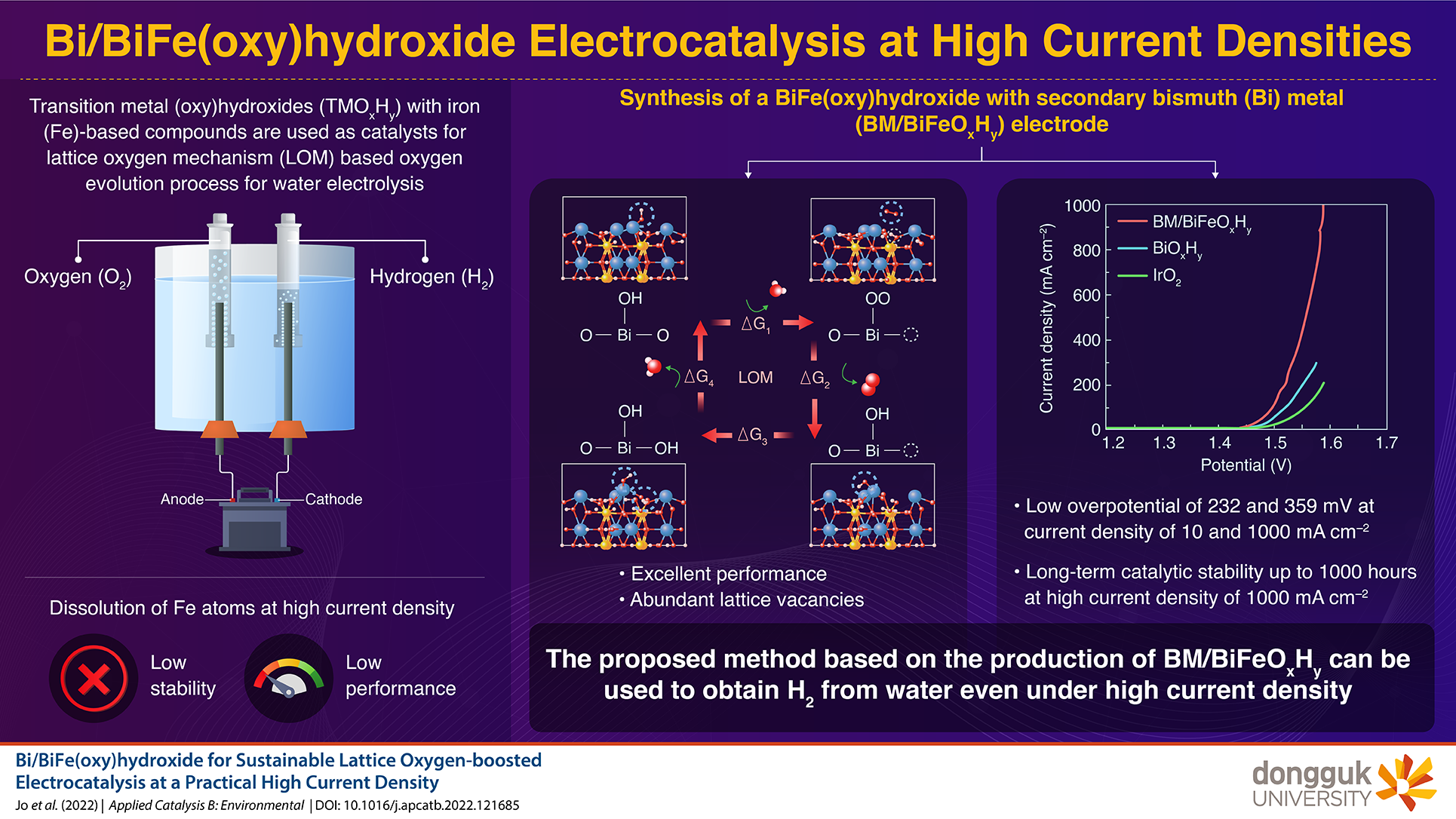
Image Credit: Dongguk University
Electrolysis of water is an efficient and sustainable method for the production of hydrogen. It involves two main reactions—the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Notably, materials used as catalysts for the OER are expensive, and demonstrate poor efficiency and stability. Recent studies have indicated lattice oxygen mechanism (LOM) as a potential pathway to increase the speed and efficiency of the OER. However, LOM uses transition metal (oxy)hydroxides (TMOxHy) with iron (Fe)-based compounds as catalysts. This is not ideal, because the dissolution of Fe atoms results in the loss of long-term stability and performance, especially under high current density.
To address this gap, a team of scientists led by Professor Jung Inn Sohn from Dongguk University investigated a new bismuth (Bi) metal (BM)-based catalyst for LOM. “The water electrolysis technology is a promising way for sustainable hydrogen generation. However, stable OER performance at a practical and high-level current density requires a new catalyst material with high performance and durability,” says Prof. Sohn while discussing the team’s motivation behind the study. Their research findings were published in Volume 315 in Applied Catalysis B: Environmental on November 15, 2022.
First, the team proposed the synthesis of a Fe-introduced Bi-(oxy)hydroxide-based electrocatalyst, which was prepared through solvothermal synthesis at 140 ℃ for 12 hours. The controlled reduction of the synthetic intermediates allowed for the formation of amorphous BM/BiFeOxHy with abundant lattice vacancies. The team found that the BM/BiFeOxHy electrode had low overpotentials of 232 and 359 mV at a current density of 10 and 1000 mA cm-2.
Further analysis also validated the long-term stability pf BM/BiFeOxHy. The team attributed this to the balanced hybridization of Bi/Fe-O, resulting in catalytic stability for up to 1000 hours at a current density of 1000 mA cm-2, without the dissolution of Fe atoms! “Our report, containing a truly promising OER candidate with an efficient catalytic performance and stability, will have an impact on the rapid development of advanced electrocatalysts for water electrolyzers,” concludes Prof. Sohn.
We are certain that soon enough, we will witness this catalyst being used in zero-emission hydrogen fuel cells!