The proposed encircling method can facilitate the development of novel, high-performing materials of both physical and biomedical relevance. Image Credit: Tokyo Tech
Current solubilization techniques often rely on time-consuming and expensive side-chain insertion into the polymer backbone, resulting in undesirable property changes.
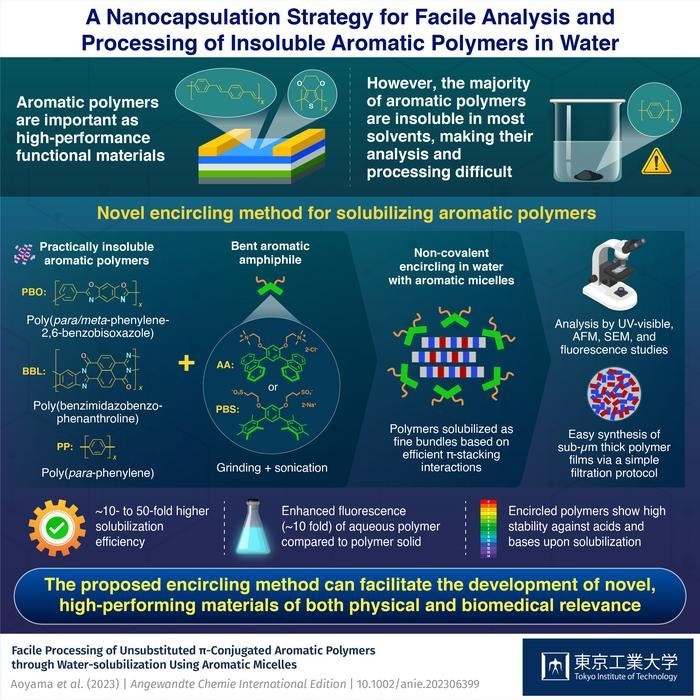
Japanese researchers at Tokyo Tech’s Laboratory for Chemistry and Life Science investigated the water-solubilization of such hard-to-handle, insoluble aromatic polymers using bent aromatic amphiphiles (i.e., surfactants) to solve this issue.
Shinji Aoyama, Lorenzo Catti, and Michito Yoshizawa demonstrate that anthracene-based amphiphile AA and its pentamethylbenzene derivative PBS, developed in their lab, have the potential of solubilizing a variety of insoluble aromatic polymers in water with high-efficiency through encircling. The study was published in Angewandte Chemie on June 5th, 2023.
AA and PBS are examples of a novel family of amphiphiles that have two hydrophilic side chains attached to a hydrophobic bent aromatic framework. These aromatic amphiphiles could enclose a wide range of hydrophobic molecules in nanocapsules that resemble micelles in water.
The polymer and aromatic amphiphile underwent an easy grinding-sonication treatment to become water-soluble. The developed clear aqueous solutions clearly displayed additional absorption bands from the solubilized polymers upon UV-visible examination.
The methodology’s high degree of generality enabled the solubilization of several types of polymers, including the notoriously intractable poly(para-phenylene) (PP), poly(benzimidazobenzophenanthroline) (BBL), and poly(para-phenylene-2,6-benzobisoxazole) (PBO; Zylon™).
The efficacy of water solubilization was found to be more than 50 times greater under the same conditions as that with conventional amphiphiles (SDS and DTAC).
Besides efficient water-solubilization, we were surprised to find that the resultant PP solution exhibits unprecedented strong bluish fluorescence (30% quantum yield (FF)). While PP shows only weak fluorescence in the solid state, the fluorescence could be increased by a factor of ~10 using our nanocapsulation strategy.
Dr. Lorenzo Catti, Assistant Professor, Tokyo Institute of Technology
Atomic force microscopy (AFM) was used to analyze the solubilized polymers’ structural composition. Combining the observed results with molecular modeling investigations, it was shown that the polymers were soluble as thin bundles (~1 nm in thickness) encircled by aromatic amphiphiles.
Notably, an easy filtering approach allowed for the aqueous processing of the solubilized polymers into thin films. Scanning electron microscopy (SEM) demonstrated that the obtained sub-micrometer-thick films are made of a compact network of closely intertwined polymer fibers.
Access to multicomponent polymer films, such as PBO-BBL, was also made possible by filtering mixed polymer solutions. The recovered amphiphiles can be used multiple times, which increases the method’s sustainability.
The study team concluded, “While the focus of this study was placed on 1D polymers, we are convinced that the generality of the method will allow us to process of a wide range of 2D and 3D polymers in the future. Doping and processing of these polymers is expected to contribute to the development of unprecedented aromatic materials.”
Journal Reference
Aoyama, S., et al. (2023) Facile Processing of Unsubstituted π-Conjugated Aromatic Polymers through Water-solubilization Using Aromatic Micelles. Angewandte Chemie. doi:10.1002/anie.202306399