Image Credit: Tokyo University of Science
Even though it is difficult to overstate the transformative impacts that LIBs have had on modern energy storage technology, they have a fair share of drawbacks that cannot be neglected anymore.
These include the restricted availability of lithium and also safety and environmental concerns. These flaws have driven researchers across the world to look for alternative battery technologies, like aqueous batteries.
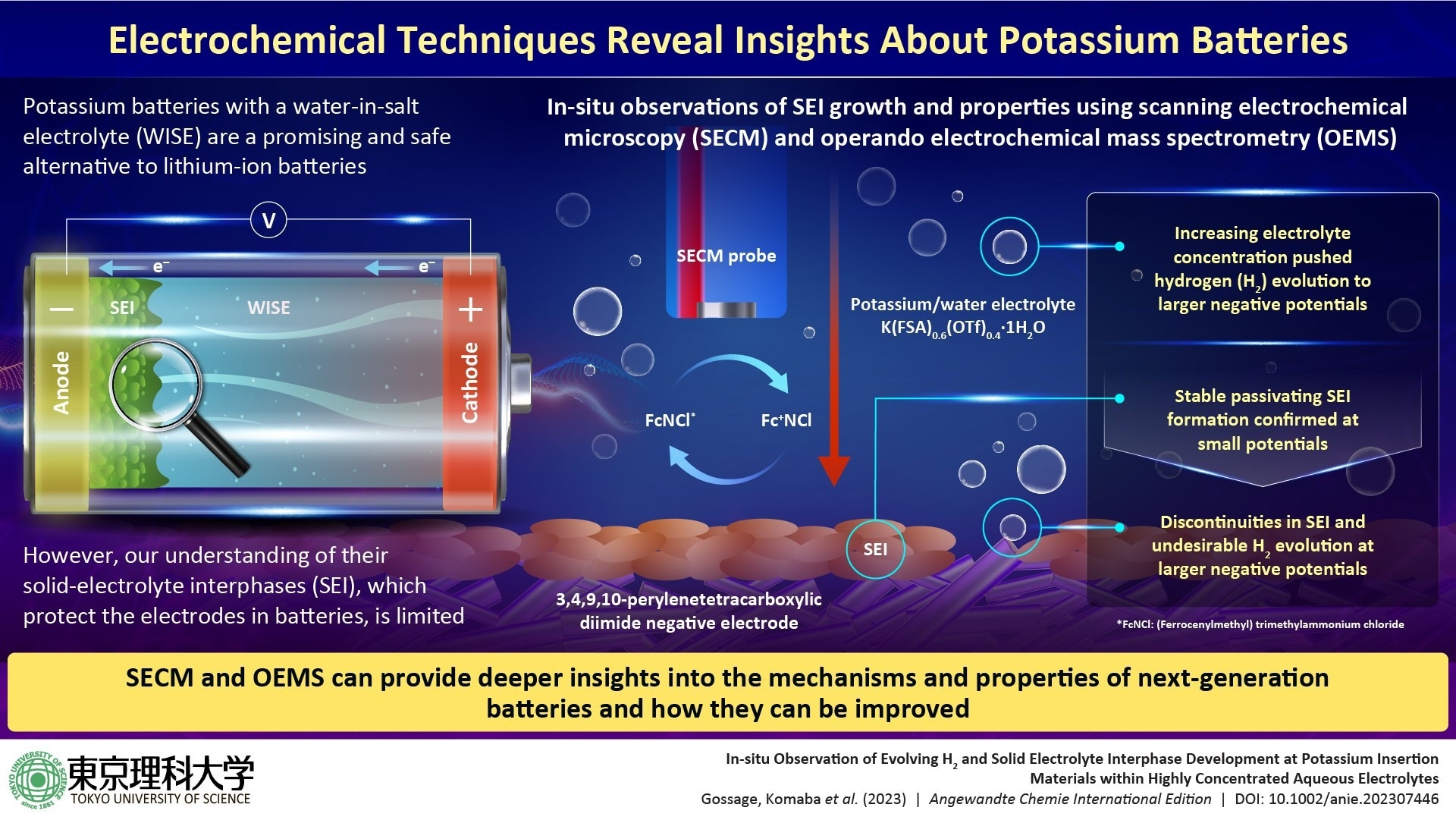
Potassium-ion batteries (KIBs) are known as a notable example; these batteries are composed of profusely available materials and are much safer compared to the LIBs. Furthermore, KIBs can use a water-in-salt electrolyte (WISE), which makes them highly stable in a thermal and chemical way.
Nevertheless, a significant challenge in the stability of high-voltage aqueous batteries lies in inhibiting hydrogen evolution at the negative electrode. In lithium-ion batteries (LIBs), solid-electrolyte interphases (SEI) play a crucial role in stabilizing the electrodes by preventing electrolyte decomposition and battery self-discharge. However, there has been limited research on SEIs in the context of KIBs.
For this major knowledge gap to be fulfilled, a research group from Tokyo University of Science- (TUS), Japan, has recently performed a pioneering study to gain knowledge into SEI formation and their properties in WISE-based KIBs.
Their study outcomes were published online in the journal Angewandte Chemie International Edition on August 18th, 2023. The study, headed by TUS Professor Shinichi Komaba, is co-authored by Junior Associate Professor Ryoichi Tatara, Dr. Zachary T. Gossage, and Ms. Nanako Ito, all from TUS.
The scientists primarily applied two improved analytical methods― operando electrochemical mass spectrometry (OEMS) and scanning electrochemical microscopy (SECM)―to note how SEI develops and reacts in real-time at the time of the operation of a KIB with a 3,4,9,10-perylenetetracarboxylic diimide negative electrode and 55 mol/kg K(FSA)0.6(OTf)0.4∙1H2O, a WISE developed by the team in an earlier study.
The experiments performed disclosed that SEI develops a passivating layer in WISE similar to that seen in LIBs, with slow apparent electron transfer rates, aiding in suppressing hydrogen evolution.
This could guarantee higher durability and stable performance of KIBs. However, the scientists noted that the coverage of the SEI layer was imperfect at higher operating voltages, thereby resulting in hydrogen evolution.
All things considered, the study outcomes demonstrated the need to explore possible avenues to improve SEI formation in future aqueous batteries.
While our results reveal interesting details on the properties and stability of SEI found in one particular WISE, we should also focus on reinforcing the SEI network to achieve improved functionality.
Shinichi Komaba, Professor, Tokyo University of Science
Komaba added, “SEI could perhaps be improved by the development of other electrolytes that produce unique SEIs, but also through the incorporation of electrolyte additives or electrode surface pretreatment.”
Also, this study emphasizes the power of SECM and OEMS for obtaining a solid knowledge of electrode-electrolyte interactions in next-generation batteries.
These techniques provide a powerful means for tracking the development, coverage, ion transfer, and stability of SEI and can easily be adapted for a variety of electrolytes and electrodes.
Shinichi Komaba, Professor, Tokyo University of Science
Komaba added, “We hope that this work encourages other researchers to further explore SECM and OEMS as advanced characterization methods that can be incorporated with traditional battery measurements to gain deeper insights.”
The development of aqueous batteries like KIBs will be considered to be instrumental for sustainable societies in the future, as they could replace the costly and dangerous LIBs that have been currently utilized in smart grids, electric vehicles, renewable energy systems, and marine applications.
Journal Reference
Gossage, Z. T., et al. (2023) In situ Observation of Evolving H2 and Solid Electrolyte Interphase Development at Potassium Insertion Materials within Highly Concentrated Aqueous Electrolytes. Angewandte Chemie International Edition. doi.org/10.1002/anie.202307446.