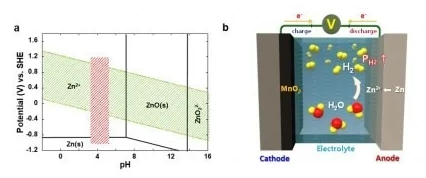
Causes of hydrogen generation and incessant accumulation within the cell in the aqueous rechargeable batteries. Image Credit: Korea Institute of Science and Technology
A research team, led by Dr. Si Hyoung Oh from the Energy Storage Research Center at the Korea Institute of Science and Technology (KIST), has successfully developed a highly safe aqueous rechargeable battery that can serve as a cost-effective and secure alternative. While aqueous rechargeable batteries may have lower energy density, they offer a significant economic advantage due to lower raw material costs compared to lithium-ion batteries (LIBs).
However, a persistent issue with aqueous batteries is the generation of hydrogen gas through parasitic water decomposition, leading to a gradual increase in internal pressure and eventual depletion of the electrolyte. This poses a substantial safety risk and has hindered commercialization efforts.
Up until now, researchers have attempted to address this challenge by implementing a surface protection layer designed to reduce the contact area between the metal anode and the electrolyte. Nonetheless, in most instances, the corrosion of the metal anode and the concurrent decomposition of water within the electrolyte are almost unavoidable. This continuous buildup of hydrogen gas can potentially lead to explosive incidents during long-term operations.
To address this pressing concern, the research team created a composite catalyst comprising manganese dioxide and palladium. This catalyst has the unique capability to automatically convert hydrogen gas generated within the cell into water, thereby ensuring both the cell's performance and safety.
Under normal conditions, manganese dioxide does not interact with hydrogen gas. However, with the addition of a small amount of palladium, the catalyst efficiently absorbs hydrogen, converting it back into water. In the prototype cell equipped with these innovative catalysts, the internal pressure remained significantly below the safety threshold, and no depletion of the electrolyte was detected.
The outcomes of this research effectively resolve a major safety concern associated with aqueous batteries, representing a significant advancement toward their potential commercial use in energy storage systems (ESS) in the future. The replacement of expensive and potentially unsafe lithium-ion batteries (LIBs) with more affordable and secure aqueous batteries has the potential to stimulate rapid growth in the global ESS market.
This technology pertains to a customized safety strategy for aqueous rechargeable batteries, based on the built-in active safety mechanism, through which risk factors are automatically controlled. Moreover, it can be applied to various industrial facilities where hydrogen gas leakage is one of major safety concerns (for instance, hydrogen gas station, nuclear power plant etc) to protect public safety.
Dr. Si Hyoung Oh, Korea Institute of Science and Technology