Comparison of Cathode Volume Changes in All-Solid-State Cells Under Low-Pressure Operated. Image Credit: Korea Institute of Science and Technology
These batteries utilize solid components, including the electrolyte, anode, and cathode, eliminating the need for a liquid electrolyte and reducing the risk of explosion.
However, sustaining the high pressure (tens of MPa) required for stable operation poses challenges, affecting factors such as energy density and capacity. The development of efficient and reliable devices for stable operation is vital for the successful commercialization of all-solid-state batteries.
Dr Hun-Gi Jung and the Energy Storage Research Center team at the Korea Institute of Science and Technology (KIST) have made a breakthrough by pinpointing degradation factors that lead to swift capacity decline and reduced lifespan in all-solid-state batteries operating under pressures comparable to those of lithium-ion batteries.
Unlike earlier research, their study is the first to verify that degradation can manifest both internally and externally in the cathode. This discovery suggests the potential for future reliable operation of all-solid-state batteries, even in low-pressure environments.
In all-solid-state batteries, the cathode and anode experience volume changes with repeated charging and discharging, leading to interfacial degradation-like side reactions and diminished contact between active materials and solid electrolytes. This escalation raises interfacial resistance, deteriorating overall cell performance. External devices are employed to counter this by sustaining high pressure, but this compromises energy density due to increased battery weight and volume. Presently, research is focusing on internal modifications within the all-solid-state cell to uphold cell performance even in low-pressure environments.
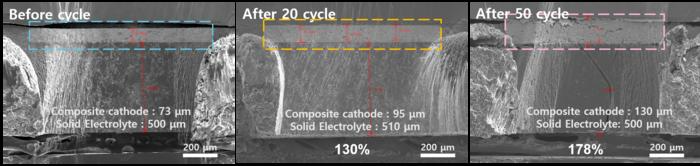
The research team conducted a detailed analysis of performance degradation by subjecting a coin-type all-solid-state battery with a sulfide-based solid electrolyte to repeated charge-discharge cycles in a low-pressure environment of 0.3 MPa, similar to that of a coin-type Li-ion battery.
After 50 cycles, the NCM cathode layer had expanded in volume by about two times, and cross-sectional image analysis confirmed severe cracks between the cathode active material and the solid electrolyte. This highlighted that, in addition to interfacial contact loss, the cracking of the cathode material and irreversible cathode phase transformation contribute to degradation in low-pressure operation.
In addition, after replacing the lithium in the cathode with an isotope (6Li) to distinguish it from the lithium present in the solid electrolyte, the team used time-of-flight secondary ion mass spectrometry (TOF-SIMS) to identify for the first time the mechanism by which lithium consumption in the cathode contributes to the overall cell capacity reduction.
During repeated charge-discharge cycles, sulfur, a decomposed product of the solid electrolyte, infused the cracks in the cathode material to form lithium sulfide, a byproduct that is non-conductive. This depleted the active lithium ions and promoted cathode phase transformation, reducing the capacity of the all-solid-state batteries.
These analytical methods, by pinpointing the degradation caused in low-pressure conditions for all-solid-state batteries, offer a promising lead in addressing their inferior cycling performance compared to conventional lithium-ion batteries.
Successfully resolving this issue holds the potential to stabilize the economic viability of all-solid-state batteries by eliminating external auxiliary devices, a primary contributor to escalating production costs.
For the commercialization of all-solid-state batteries, it is essential to develop new cathode and anode materials that can be operated in a pressure-free or low-pressure environment rather than the current pressurized environment. When applying low-pressure-working all-solid-state batteries to medium and large-scale applications such as electric vehicles, it will be expected to make full use of established lithium-ion battery manufacturing facilities.
Dr. Hun-Gi Jung, Energy Storage Research Center, Clean Energy Research Division, Korea Institute of Science and Technology
Journal Reference
Shin, H., et al. (2023). New Consideration of Degradation Accelerating of All-Solid-State Batteries under a Low-Pressure Condition. Wiley Online Library. doi/10.1002/aenm.202301220