By converting air into fertilizer, scientists at Stanford University and the Department of Energy’s Oak Ridge National Laboratory are reducing carbon emissions. Their research could offer a crucial answer to achieving global carbon-neutral targets by 2050.
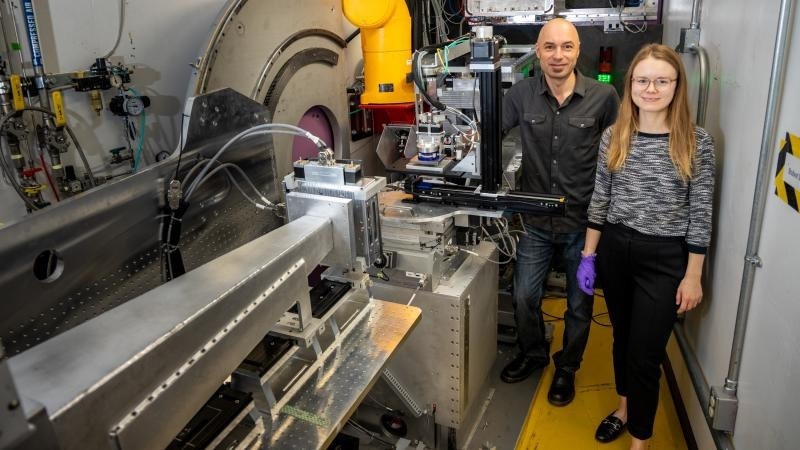 Mat Doucet, left, of Oak Ridge National Laboratory and Sarah Blair of the National Renewable Energy Lab used neutrons to understand an electrochemical way to produce ammonia. Image Credit: Genevieve Martin/ORNL, US Dept. of Energy
Mat Doucet, left, of Oak Ridge National Laboratory and Sarah Blair of the National Renewable Energy Lab used neutrons to understand an electrochemical way to produce ammonia. Image Credit: Genevieve Martin/ORNL, US Dept. of Energy
The study, which was published in the Royal Chemical Society’s Energy & Environmental Science, outlined a sustainable electrochemical method as an alternative to a chemical one for creating ammonia, which is a necessary component of nitrogen fertilizer.
Essentially, the researchers employed neutron scattering to comprehend how the nitrogen reduction reaction—a process that converts nitrogen to ammonia—increases the quantity of ammonia generated by cycling an electric current.
With the help of this method, farmers may be able to create fertilizers based on ammonia without releasing carbon dioxide, even though nitrogen is the most prevalent element in the environment.
Ammonia is critical to food supplies for most of the world’s population. As the world population continues to grow, we need sustainable ways to produce fertilizers — especially as warming intensifies.
Sarah Blair, Postdoctoral Researcher, National Renewable Energy Laboratory
Farmers can grow more food in less area by using industrial fertilizers. Despite this, the Haber-Bosch process, which has been used to produce industrial ammonia for over a century, accounts for almost 2% of total CO2 emissions due to the fossil fuels required. Two percent might not seem like much, but we are adding carbon dioxide to the atmosphere faster than the earth can absorb it, so every effort must be made to reduce that figure.
The Haber-Bosch process emits around 500 million tons of carbon dioxide each year, which would need the equivalent of practically all federal lands in the United States to collect and store.
The study’s findings could help scientists understand alternative ways of producing carbon-neutral ammonia for various purposes. These might include recycling or recapturing fertilizer runoff before it enters waterways, as well as manufacturing ammonia at seaports for ship fuel. Global shipping contributes another 3% of global CO2 emissions, while fossil fuel burning is the major source of CO2 from human activity.
Blair added, “You can’t improve the design of something if you don’t know how it is already working. Neutrons help science evolve by shedding light at the atomic level on certain systems that are impossible to study otherwise.”
The Liquids Reflectometer at the Spallation Neutron Source was used for Blair's neutron studies, together with senior ORNL neutron scattering scientist Mat Doucet. Their goal was to comprehend the impact of alternating an electric current on the solid-electrolyte interface (SEI) development in a nitrogen reduction reaction system that uses lithium as a mediator to make ammonia.
Comprehending the creation of SEIs is essential for improving battery performance and understanding the physics underlying the electrochemical generation of ammonia. Additionally, this study is the first time that the creation of an SEI layer during this specific electrochemical conversion has been seen using neutron-based methods.
Furthermore, the investigation yielded a unique new neutron method called time-resolved reflectometry. By slicing neutron data into intervals of a few seconds, scientists can record more detail, akin to viewing a movie frame by frame.
Blair and Doucet first believed that the electrochemical changes they saw were gradual. However, because of the new method, scientists were able to find changes occurring at considerably smaller time intervals.
Processes that appear to be linear might not be linear at all when you look at them more closely. Getting to that structure as a function of time is the hard part. The technique we developed for this experiment allowed us to do just that.
Mat Doucet, Senior Neutron Scattering Scientist, Oak Ridge National Laboratory
Scientific discoveries made at SNS provide the groundwork for technical breakthroughs that enhance people’s daily lives. For SNS users, the method Blair and Doucet created opens up new avenues in electrochemistry.
These time-dependent experiments will draw scientists who study separation chemistries.
Hanyu Wang, Instrument Scientist, Oak Ridge National Laboratory
ORNL Neutron Reflectometry group leader Jim Browning added, “Their approach can answer a lot of questions for separation chemistries, batteries and an entire gamut of different areas of interest, like energy production, energy storage and conservation of energy.”
SNS, a user facility of the DOE Office of Science, provided resources for this study.
Journal Reference:
Blair, S. J., et. al. (2023) Combined, time-resolved, in situ neutron reflectometry and X-ray diffraction analysis of dynamic SEI formation during electrochemical N2 reduction. Energy & Environmental Science. doi:10.1039/D2EE03694K