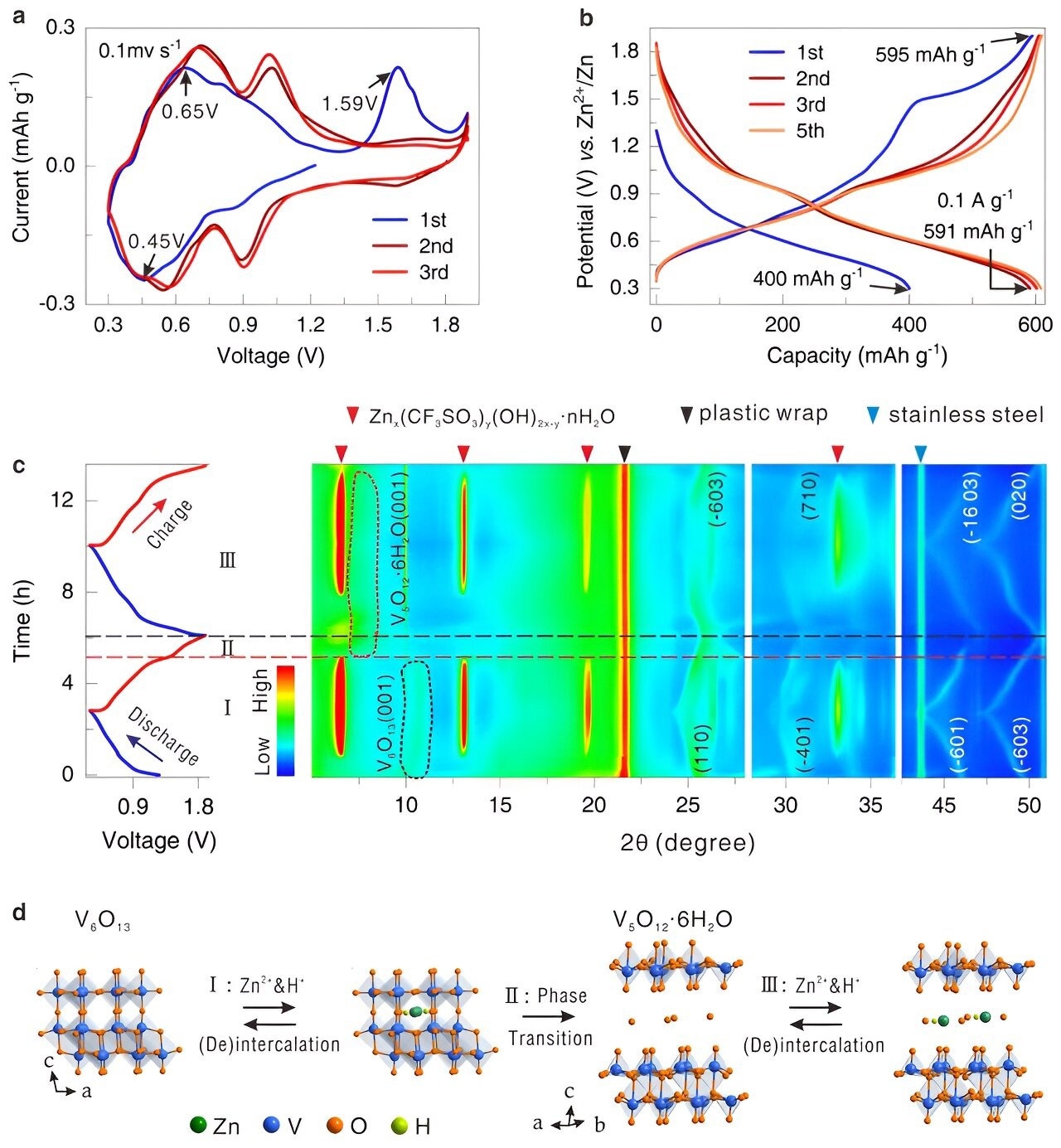
The electrochemically induced phase transformation behaviors of the V6O13 cathode. Image Credit: Mo Li’e
A promising technology for large-scale stationary energy storage is aqueous zinc-ion batteries (AZIBs). To increase the technology’s commercial viability, scientists have created novel cathode materials that enhance performance.
For AZIBs, vanadium oxides (VOx) are generally regarded as a good choice. However, proving VOx’s dominance has proven difficult due to their slow Zn2+ diffusion kinetics and low electronic conductivity.
In this work, high-performance aqueous zinc ion cathode materials are produced by building an in situ electrochemically induced phase transition.
A unique procedure was employed to convert the structure of a material known as V6O13 to V5O12·6H2O upon initial charging. This modification increased the material's capacity to store energy by improving its conductivity of electricity and facilitating the movement of zinc ions.
In addition, the modified material featured spaces that facilitated particle movement and maintained its stability throughout numerous cycles of charging. Consequently, the new material exhibited high-energy storage capacity, rapid charging times, high performance at high charging rates, and extended battery life without degrading.
The team claims that this new approach offers a fresh perspective on how to overcome the difficulties involved in creating high-performance cathodes for AZIBs.
Journal Reference
Mo, Li’e., et al. (2023). Electrochemically Induced Phase Transformation in Vanadium Oxide Boosts Zn-Ion Intercalation. ACS Nano. doi/10.1021/acsnano.3c11217.