Reviewed by Lexie CornerFeb 23 2024
Under the direction of Professor Huijuan Liu, a research team at Tsinghua University has created a novel electrochemical system that holds the potential to transform the recovery of metal from industrial effluent. Engineering published the research.
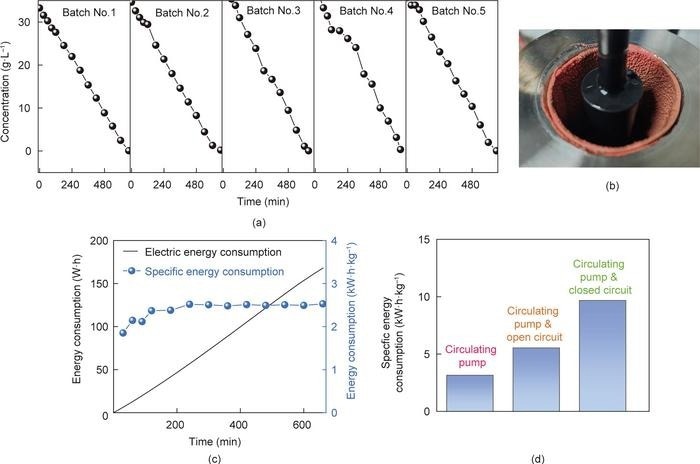 (a) Cu2+ concentration change under different electrodeposition batches, (b) image of Cu recovered from TE&SF electrodeposition, (c) electric energy and specific energy consumption during electrodeposition, and (d) energy consumption of the entire process. Image Credit: Li Chen et al.
(a) Cu2+ concentration change under different electrodeposition batches, (b) image of Cu recovered from TE&SF electrodeposition, (c) electric energy and specific energy consumption during electrodeposition, and (d) energy consumption of the entire process. Image Credit: Li Chen et al.
Since industrial effluent contains heavy metals, it presents serious environmental risks. Existing metal recovery techniques, such as electrodeposition, are hindered by interfacial ion transport, which makes recovery sluggish and of poor quality. The group in the study put out a unique strategy that simultaneously enhances mass transfer and fosters interfacial ion transport by integrating a transient electric field (TE) and swirling flow (SF).
The study group investigated how various operational parameters, such as flow rate, transient frequency, and operation mode, affected metal recovery. They found that the ideal parameters—low and high electric levels of 0 and 4 V, 50 % duty cycle, 1 kHz frequency, and 400 L/h flow rate—were reached for the fastest and most effective sequential recovery of copper in TE&SF mode.
It was discovered that the kinetic coefficients of TE&SF electrodeposition were 1.37−1.97 and 3.5−4.3 times, respectively, greater than those of single TE and SF electrodeposition.
The researchers simulated the deposition process under TE and SF circumstances to obtain insights into the procedure. The outcomes demonstrated the effective cooperation of charge transfer and interfacial ion transport, resulting in quick and high-quality metal recovery. The mixed deposition approach encourages resource recycling and effectively reduces metal pollution.
This novel strategy addresses the shortcomings of interfacial ion transport in traditional electrodeposition techniques. The group effectively enhances the kinetics of the reaction by improving the bulk and interfacial ion transport by connecting a transient electric field with turbulent flow. Together, the transitory electric field and swirling flow produce deposits with uniform morphologies and homogenous compositions, as well as quick metal recovery.
The system also has broad application in recovering metals with redox potentials higher than water reduction and hydrogen evolution. This ability greatly benefits enterprises handling metal waste since it makes it possible to recover precious and heavy metals at high values.
Professor Huijuan Liu and her colleagues’ study provides fresh perspectives on effective metal recovery from industrial effluent. Their discoveries pave the way for resource- and environmentally-efficient metal recycling techniques that will help save precious resources and lessen pollution.
Journal Reference:
Chen, L., et. al. (2024) Toward Efficient Metal Recovery from Industrial Wastewater: An Electrochemical System in Potential Oscillation and Turbulence Mode. Engineering. doi:10.1016/j.eng.2023.12.002