In a recent paper published in The European Physical Journal D, researchers from Johannes Gutenberg University Mainz, Germany, have demonstrated a method for more precise ionization measurements of elements. This advancement is particularly significant for enhancing the detection and remediation of radioactive waste.
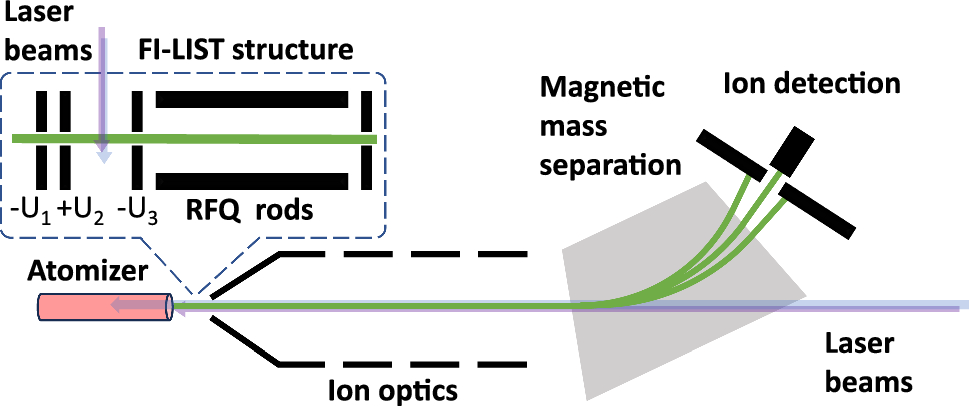 The mass separator used in the experiments. Green: ion trajectory; blue and violet, laser beams. Image Credit: EPJ, Kaja et al.
The mass separator used in the experiments. Green: ion trajectory; blue and violet, laser beams. Image Credit: EPJ, Kaja et al.
Neptunium, a principal radioactive component of nuclear waste, has a complex atomic structure that can be explored through mass spectrometry. This analysis is crucial both for understanding its inherent properties and for determining the isotope composition of neptunium waste. Magdalena Kaja and her team at Johannes Gutenberg University in Mainz, Germany, have developed a novel laser spectroscopy technique that allows for more precise measurements of neptunium's ionization potential than previous methods.
As an actinide metal positioned next to uranium on the Periodic Table, neptunium has an atomic number of 93. Its name, inspired by the planet Neptune—situated beyond Uranus in the Solar System—is a nod to its position. Of its 25 known isotopes, most are extremely short-lived. However, the most stable isotope, neptunium-237 (237Np), has a half-life exceeding 2 million years, making it a particularly hazardous nuclear contaminant.
The samples of neptunium isotopes available for this type of analysis are tiny: they generally comprise only a few atoms of an isotope.
Multistep resonance ionization using a laser source has been proven to be the most useful technique for this, providing high sensitivity, specificity, and precision.
Magdalena Kaja, Johannes Gutenberg University
Magdalena Kaja and her colleagues utilized a cutting-edge setup featuring a solid-state titanium: sapphire laser system, an enhanced laser ion source, and a high transmission mass separator. This advanced apparatus was instrumental in their research on neptunium.
The team employed this technology to measure the first ionization energy of neptunium, which is the energy required to remove the first electron from its outermost electron shell, thereby forming a positive ion. They precisely determined this value to be 6.265608(19) eV. This measurement not only aligns with previously reported values in scientific literature but also achieves a level of precision more than ten times greater than any prior measurements.
We now aim to extend our investigations to rare neptunium isotopes.
Magdalena Kaja, Johannes Gutenberg University
The methods can also be applied to the analysis and detection of neptunium traces in radioactive waste.
Journal Reference:
Kaja, M., et al. (2024) Resonant laser ionization of neptunium: investigation on excitation schemes and the first ionization potential. The European Physical Journal D. doi.org/10.1140/epjd/s10053-024-00833-7.