Introduction
The Langmuir-Blodgett (LB) method can provide organized molecular assemblies with well-defined molecular orientation and ordered layer structure which have been widely applied to various functional devices [1]. Conventional Langmuir-Blodgett (LB) films were prepared from low-molecular-weight amphiphilic compounds. These LB films, however, have poor stability with respect to mechanical and thermal treatments, and poor resistance to the dissolution of organic solvents. Recently, polymer LB films have been extensively investigated in order to overcome these draw-backs [2, 3]. In many pervious studies, it has been found that poly N-dodecylmethlacrylamide (pDDMA) can form a stable monolayer on the water surface, and a stable LB film [4]. 1, 4-dioxaspiro [4, 4] nonane-2-methyl methacrylate is a type of ketal-protected substituted group, as an acid labile protective group which generates cyclopentanone in the exposed region [5]. Our goal in this investigation was to synthesize a copolymer of these two kinds of substituents for a deep UV chemically amplified resistant system.
We know well that the behavior of Langmuir monolayer is greatly important for understanding the structure, stability and deposition transfer ratio of monolayer. By measuring and analyzing the ∏- A isotherm and elasticity of monolayer, one would be able to understand the state of monolayer and its changes, for example, molecular arrangement, phase structure, and phase transition etc. The elasticity of monolayer can be defined as E=-A∆σ/∆A =-A d∏/dA, where ∏=σ0-σ, and σ0 is the surface tension in the absence of monolayer, ∏ the surface pressure and A the mean molecular area. The static elasticity (or compressing modulus) can be directly calculated from the slope of ∏-A isotherm [6].
In this study, a new copolymer is prepared with N-dodecylmethlacrylamide (DDMA) and 1, 4-dioxaspiro [4, 4] nonane-2-methyl methacrylate and was spread successfully on the air/water interface. The characteristics of Langmuir film of the copolymer were investigated and the molecular orientation of the copolymer was discussed. It is expected that further work on the compounds could corroborate its potential as photo resist for photolithography.
Experimental
N-dodecylmethlacrylamide (DDMA) was synthesized from α-methacryloyl chloride with dodecylamine in the presence of triethylamine in chloroform at 00C (Scheme1). The crude product was purified by column chromatography (100---200 mesh of gel, eluted: acetic ether / petroleum ether = 1/ 5 or dichloride methane). A colorless crystal was obtained after removing solutions and drying under vacuum overnight. The yield was 83%, m.p.:37—380C. The 1H NMR data was given as follows: (CDCl3 ppm) δ0.86-0.9 (t.3H), 1.26—1.30 (d, 18H), 1.49-1.57 (q, 2H), 1.96 (s, 3H), 3.28—3.33 (q, 2H), 5.30-5.31 (d, 1H), 5.66 (s, 1H), 5.80 (s, 1H). FT-IR (cm-1): 3330-3050(υ N-H), 1653 (υC=O), 1607 (υC=C), 1534(δN-H), 1473(υC-N). Element analysis: C%: 75.32 (75.83), H%: 12.45 (12.37), N%: 5.34 (5.53).
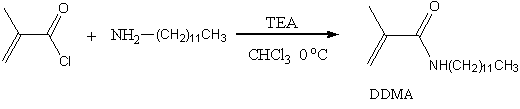
Scheme 1. Synthesis and structure of DDMA.
1, 4-dioxaspiro [4, 4] nonane-2-methanol (DNMM) was synthesized from glycerol with cyclopentanone in the present of FeNH4 (SO4)2 as a catalyst in cyclohexane at 70 0C (Scheme2). Colorless oil was produced by distilling under reduced pressure. The 1H NMR data was given as follows: (CDCI3 ppm):δ 4.0-4.1 (m, 1H),3.8-3.9 (m, 1H), 3.7 (m, 1H), 3.5-3.6 (m,2H), 2.6 (t,1H), 1.6-2.0 (m,8H). IR (cm-1): 3454(υ-OH), 2939(cyclic C-H), 1336, 1088(υ C-O ).
1.4-dioxaspiro [4.4] nonane-2-methyl methacrylate (DNMMA) was synthesized from DNMM with α-methacryloyl chloride and dodecylamine in the presence of triethylamine in THF under dry nitrogen atmosphere at 00C for 8 h (Scheme2). After filtration, the reaction mixture was washed twice with NaHCO3 aqueous solution and distilled water, respectively. The organic layer, which was extracted with ethyl ether and dried with anhydrous MgSO4, was filtrated, concentrated, and dried under vacuum overnight. The 1H NMR data was given as follow: CDCI3 ppm):δ 6.1(s,1H), 5.6 (s,1H), 4.3 (m,1H), 4.2 (d,2H), 3.7(m,1H), 4.0(m,1H), 1.9(s,3H), 1.4-1.8(m,8H). IR (cm-1): 2960(υ cyclic C-H), 1719(υ C=O), 1638(υ c=c), 1336, 1108(υ C-O).
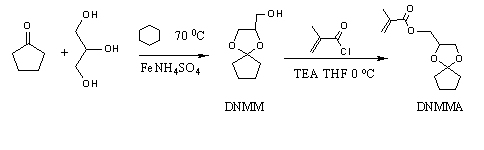
Scheme 2. Synthesis and structure of DNMMA.
Poly (N-dodecylmethlacrylamide-co-1, 4-dioxaspiro [4, 4] nonane-2-methylmethacrylate) p (DDMA- DNMMA) was prepared by free-radical copolymerization in toluene at 600C (Scheme3), using 2, 2’ –azobis isobutyronitrile(AIBN) as a thermal initiator. The copolymer was purified by re-precipitation from a large excess of hexane, and dried under vacuum at room temperature. The ratio of DDMA to the molecular unit was 13 mol% (determined by 1H NMR).
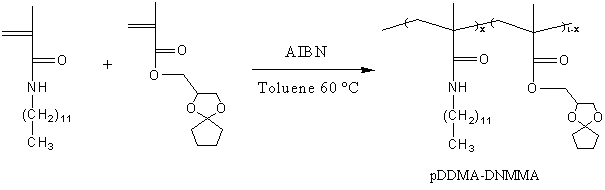
Scheme 3. Synthesis and structure of pDDMA-DNMMA.
Measurements of the surface pressure (∏) versus average repeating unit area (A) were performed on a fully automated Langmuir-type film balance (KSV 5000, KSV Instruments, Helsinki, Finland). A Wilhelmy plate is used as the surface pressure sensor and situated in the middle of the trough. Two barriers compress or expand symmetrically at the same rate from two sides of the trough. Distilled and deionized water with resistant values higher than 18.2 MΩ (MILIPORE, MILI-Q gradient) was used for a subphase. The copolymer was dissolved in chloroform at a concentration of approximately 1.053x10-3 M and the solution was spread on the water surface by means of a micrometric syringe. After the solution of the copolymer was spread onto the surface of water, the solvent was let evaporate out for about 30 min and then the monolayer was compressed at a compression rate of 10 mm/min. The subphase temperature was controlled by water circulation from a thermostat, within an error range of ± 0.50C. The isotherms for every sample were measured for two times to ensure good reproducibility. The static elasticity (dilation modulus) can be calculated from the slope of ∏- A isotherm. The structure of the monomer and the copolymer composition were determined by FTIR and 1H NMR.
Results and Discussion
Figure 1 shows the surface pressure (∏) and static elasticity (Es) of pDDMA –DNMMA as functions of surface area (A). Curve 1, ∏-A isotherm, indicates that the copolymer can form stable monolayer, in which a steep rise in surface pressure and a relatively high collapse pressure could be observed. We can also see roughly that the gas-liquid and liquid-solid phase transitions occurred during compression, at the mean time repeating unit area of 0.76 nm2 and 0.52 nm2 were obtained, respectively. The monolayer will collapse at the surface pressure of 46 mN/m. The average molecular occupied surface area per repeating unit is estimated by extrapolating the steep rising part of ∏-A curve to zero pressure. It was obtained to be 0.52 nm2.

Figure1. The surface pressure (1) and static elasticity (2) vs. the surface area for every repeating unit.
On the other hand, the static elasticity (Es) - area (A) isotherm (curve 2) can be divided into three parts obviously. This copolymer exists in the gaseous state (G) in the area range of 1.36—0.8 nm2, which is almost a horizontal line with the Es values of about 0. When compressed further the monolayer undergoes a phase transition to the liquid-expanded state (L1), with a sharp increase of Es in a small region about 0.78 nm2. Through a coexistence region of L1 and L2, the L1 phase undergoes a further transition to the liquid-condensed state (L2). Upon further compression, the film-forming molecules become closer to each other and the intermolecular interactions become stronger. As a result, the elasticity increases distinctively until nearly the surface pressure is about 44 mNm-1 and the area is approximately 0.43 nm2; and the monolayer finally reaches the solid state (S). If the monolayer is further compressed after reaching a critical value at even higher densities, the monolayer will collapse into three-dimensional structure. This is reasonable that the copolymer monolayer on water surface has the closest molecular packing and can be considered as a two-dimensional crystal solid.
It should be noticed that there are sever peaks shown in the insert of Figure 2, which are located in the liquid-solid or solid transition region. This implies that the intermolecular interaction in pDDMA-DNMMA monolayer has some discontinuous changes and there may be some additional explicit phase transitions during compression. The monolayer of this copolymer may be developed complex domain structure in some phase, such as liquid expended-condensed coexistence region [7]. Compared with the small inflection corresponding to the phase transitions in ∏-A isotherm, the peak in Es-A curve is more obvious and provides more information about the microcosmic changes. In fact, Es-A curve is just another form to express the change of surface pressure during the compression, and can be more conveniently used for the monolayer studies (e.g. phase transition) than its integral form of ∏-A isotherm.
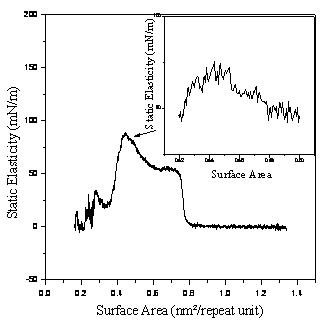
Figure2. The static elasticity vs. surface area for pDDMA-DNMMA monolayer. The insert clarifies the date of repeating unit area from 0.50 to 0.42 nm2.
While the ∏-A isotherm has only shown three possible regions, such as gas (G), liquid (L) and solid (S), the Es-A curve had several distinct phase transition regions. Not only usual gas, liquid and solid region but also an additional, almost horizontal (L1-L2) transition phase could be seen from Figure 1 curve 2. It is obvious that the Es-A curve is well defined than the ∏-A isotherm is.
The critical mean repeating unit area of 0.43 nm2 calculated from Es-A curve is relatively smaller when compared with the average repeating unit area 0.52 nm2 obtained from ∏-A isotherm. However, both numbers are greater than the values calculated from Chem. Draw of a ketal- ring (0.09nm2) with face-on orientation, or 0.28 nm2 reported by Tokuji Miyashita [8]. The difference indicated that the alkyl chains in the copolymer are tilted more than that in the pDDMA homopolymer. The schematic illustration is shown in Figure 3, and the molecular orientation is under investigation by X-ray diffraction measurement and UV absorption spectra in following work. Thus it can be seen that Es-A curve can give more precise value of mean molecular area than the ∏-A isotherm.
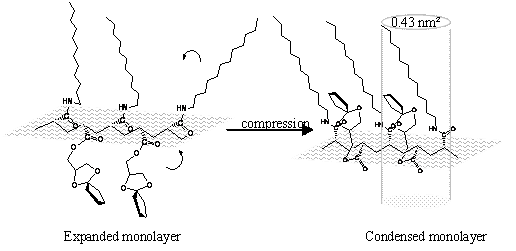
Figure 3. The probable molecules orientation of pDDMA-DNMMA in the monolayer at the air/water interface.
Conclusion
A new Copolymer, poly (N-dodecylmethlacrylamide-co-1, 4-dioxaspiro [4, 4] nonane-2-methyl methacrylate) (pDDMA-DNMMA), has been synthesized. The surface behavior of the copolymer monolayer at the air/water interface was investigated by measuring the surface pressure (∏) and the static elasticity (Es) as a function of mean repeat area (A) isotherm. The isotherm indicates that pDDMA-DNMMA can form a stable, condensed monolayer with a steep rise in ∏, and has a high collapse pressure of 46 mN/m and no much discontinuities could be observed in the ∏-A isotherm. The elasticity – Area curve of pDDMA-DNMMA shows that the interactions between macromolecules in copolymer have some discontinuous changes during compression. This implies some unusual phase transitions have occurred, which can hardly be found out from the ∏-A isotherms of the monolayer. From this point, the Es-A curve can be convinced better, more intuitionistic, and more sensitive to expose the monolayer state than ∏-A isotherm because elasticity can give more detailed information on molecular interaction and motion.
Acknowledgements
This work is supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, the Natural Science Foundation and the Innovation Fund for Outstanding Scholar of Henan Province.
References
1. A. M. Markowitz, V. Janout and G. D. Castner, “Perforated Monolayers: Design and Synthesis of Porous and Cohesive Monolayers from Mercurated Calix[n]arenas”, J. Am. Chem. Soc., 111 (1989) 8192-8200.
2. A. Laschewsky, H. Ringsdorf, G. Schmidt and J. Schneider, “Self-organization of Polymeric Lipids with Hydrophilic Spacers in Side Groups and Main Chain: Investigation in Monolayers and Multilayers”, J. Am. Chem. Soc., 109 (3) (1987) 788-796.
3. J. Schneider, H. Ringsdort and F. J. Rabolt, “Structural Studies of Polymers with Hydrophilic Spacer Groups: Infrared Spectroscopy of Langmuir-Blodgett Multilayers of Preformed Polymers with Hydrocarbon Side Chains”, Macromolecules, 22 (1989) 205-210.
4. Y. Mizuta, M. Matsuda and T. Miyashita, “Preparation of polymer Langmuir-Blodgett Films Containing Pyrene Chromophore and Energy Transfer in the Films”, Langmuir, 9 (1993) 1110-1114.
5. J. B. Kim, J. J. Park and J. H. Jang, “Chemically Amplified Resists Based on Poly(1,4-dioxaspiro[4.4]nonane-2-methyl methacrylate)”, Polymer, 41 (2000) 149-153.
6. K. Fang, G. Zou and P. S. He, “Dynamic Viscoelasticity Properties of Spread Monostearin Monolayer in the Presence of Glycine”, J. Colloid and Interface Sci., 266 (2003) 407-414.
7. P. S. He, K. Fang and G. Zou, “Elasticity of Langmuir Monolayer Detected by Dynamic Oscillation Method”, J. Colloid and Surfaces A: Physicochem. Eng. Aspects, 201(2002) 265-273.
8. Tokuji Miyashita, Fei Feng and Yutaka Amao, “A polymer Langmuir-Blodgett Film Containing Porphyrin Chromophore”, Thin solid films, 366 (2000) 255-259.
Contact Details
|
Wenjian Xu, Guoliang Zeng, Tiesheng Li and Yanjie Wu
Department of Chemistry
Zhengzhou University
Henan Key Laboratory of Chemical Biology and Organic Chemistry
Open and Key Laboratory of Applied Chemistry of Henan Higher Institution
Zhengzhou 450052
P. R. China
E-mail:[email protected]
|
Fangfang Ren and Suhua Zhang
College of Materials and Engineering
Zhengzhou University
Zhengzhou 450052
P. R China
|
|