Oct 9 2001
Wikimedia Commons
During ceramics production, one of the steps is the processing of inorganic powder suspensions. For instance, tape casting of thin layers for multi-capacitors, casting tableware body suspensions with the help of porous plaster molds, spray drying ferrite suspensions to produce a granulate for pressing or calcining, etc. are some of the processes used in ceramics production. These suspensions are usually, but not exclusively, aqueous-based.
Suspension Control
Hence, the optimization and control of suspensions represent a major step. Since suspensions are often used at the beginning of production, failure to control can lead to poor yields at each of the succeeding process steps (firing, drying, etc.). Even if yields are satisfactory, the final microstructure-dependent properties (for example, electrical properties and strength) can be severely compromised.
Rheology and Zeta Potential
Ceramic producers have traditionally employed rheological measurements for quality control of suspensions. The Brookfield, Ford Cup, and Torsion viscometers are all examples of devices that are often found in ceramic factories. Rheology typically quantifies the potential of a suspension to move as it is sheared (or undergoes the application of a force).
But rheological behavior itself is mostly defined by the extent to which the particles in suspension interrelate with one another. Although particle shape and size play a role, one major factor that governs the level of interaction is the charge present at the surface of the particle. In this case, zeta potential can be taken as a measure of this charge.
What is Zeta Potential?
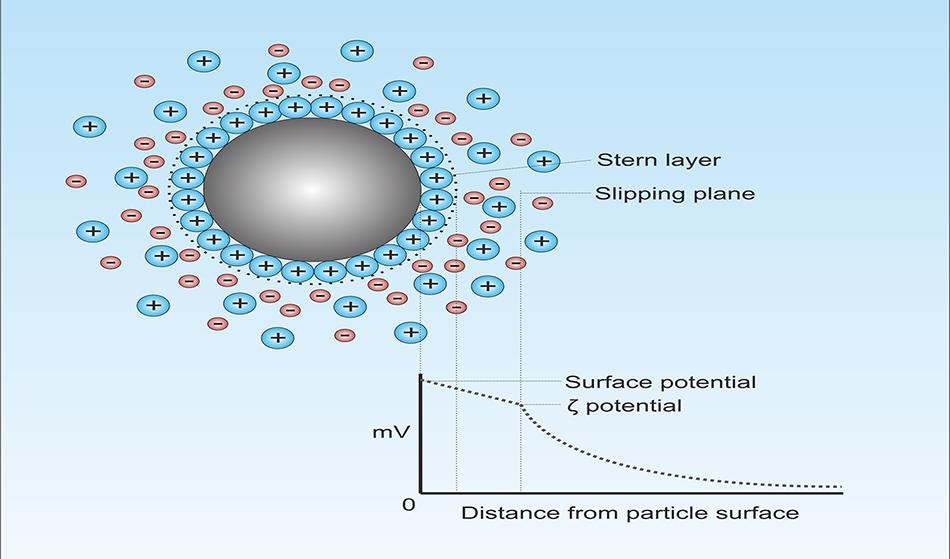
When inorganic particles are dispersed in water, they assume a charge. The same phenomenon occurs in silica because surface silanol (Si-OH) groups end up losing a proton. The aqueous phase turns out to be somewhat acidic (since it receives protons), while the surface of the silica becomes negative (because of the formation of Si-O−). The surface of the charged particle subsequently attracts a layer of counter-ions (negatively charged ions) from the aqueous phase. In the case of silica, positive ions (Na+, K+) will accumulate at the surface.
Owing to ionic radii considerations, the strong adsorption of counter-ions will not lead to the offsetting of the surface charge completely. This subsequently results in the formation of a second layer of more loosely held counter-ions. At a specific distance from the surface of the particle, counter-ions will completely balance the surface charge. Further than this point, bulk suspensions with a balance of positive and negative electrolytes exist.
Double Layers
The double layer’s size will be dependent on, first, the amount of charge present on the surface of the particle. A large charge, be it negative or positive, will lead to a large double layer that prevents particles from getting close to one another. This is because particles carrying the same electrical charge tend to have electrostatic repulsion.
This situation is characteristic of stable (deflocculated) suspensions that have a low viscosity. On the other hand, a low surface charge needs a less number of counter-ions and hence smaller double layers. As a result, particles tend to flocculate, leading to high-viscosity suspensions.
What is it a Measure of?
Zeta potential is the energy required to shear the particle as well as its internal layer of counter-ions away from the external layer/bulk medium. It is measured in millivolts.
How is it Determined?
Conventionally, zeta potential has been determined by electrophoresis. When electrodes are inserted into a suspension and a DC voltage is applied, the charged particles are pulled toward the electrode that has the opposite charge. Therefore, silica particles will travel toward the anode (positive electrode). The velocity of the traveling particles is dependent on the voltage applied and the particle surface charge (or zeta potential). Hence, if the velocity can be quantified, zeta potential can be established using the Van Smolukoski or Henry equations.
Measurement Techniques
Light scattering techniques are traditionally used for measuring the velocity of particles. While these techniques are good, they require highly dilute suspensions (possibly as low as 0.1 vol% solids loading). It can be debated that these suspensions are highly different from the high solids (40 vol%+) suspensions often found in the industry.
Produced by Dynamic Colloids, the AcoustoSizer helps in measuring zeta potential (concurrently, particle size distribution) in high solids suspensions. The AcoustoSizer does not use light measurements, but instead depends on the generation or measurement of sound waves.
How the AcoustoSizer Works
A cube-shaped cell, containing a conductivity meter, pH probe, and stirrer, is used to hold the suspension. A short pulse (several cycles) of the alternating voltage is then applied to the cell’s opposing sides through the electrodes. This makes the particles to vibrate to and fro, as they are alternately attracted and then repelled based on the electrodes’ changing polarity.
The alternate rarefaction and compression of the suspension between the cell wall and the particle, generate a sound wave that moves down the glass tube ceramics, and is quantified at the pressure transducer. This intricate sound wave is known as the electrokinetic sonic amplitude (ESA) and is a combination of responses from the particles of different sizes.
A particle size distribution plot can be produced by repeating the measurements using applied voltage pulses with increasing frequencies. This can indeed be achieved because, when the voltage frequency is increased, particles of larger size find it more and more difficult to vibrate in reaction to the alternating voltage. Hence, the particles lag further behind the polarity switches and do not move much.
Measurements and How to Use the Data
A given inorganic powder can be optimally characterized by demonstrating how the zeta potential differs as a function of pH, and not using a single-point zeta potential measurement. This, however, can create problems when producing a suspension containing alumina and silica. When the zeta vs pH plots are similar, optimal mixing of components becomes possible.
Methods for Altering Zeta Potential
It was stated before that particle charge normally influences the size of the double layer, and thus the zeta potential. When a component’s zeta potential is not satisfactory, the situation can be manipulated in several ways.
Surfactants
Firstly, surfactants can be added to the suspension. Such compounds are adsorbed onto the surface of the particles to modify the surface charge.
Surfactants can be grouped into three major categories:
- Steric surfactants—they keep particles apart by virtue of the space taken up by the organic surfactant molecules when coupled to the particle
- Electrostatic surfactants—in this case, the surface charge of the particle is purely modified by virtue of the charge existing on the adsorbed surfactant molecule
- Electrosteric surfactants—they are a combination of the above
The various surfactants as well as surfactant chemistries provide a remarkable flexibility in terms of the changing suspension behavior, to realize the most optimal conditions for the next processing step.
Soluble Ions
Secondly, the zeta potential can be modified by altering the valence and concentration of soluble ions in the suspension. For instance, if the same silica example is considered, then a single Ca2+ ion in suspension will do a relatively better job of annulling the negative surface charge on the silica when compared to a K+ or Na+ ion. This results in a double layer that is more compressed and has increased flocculation. Usually, a suspension state intermediate of deflocculation and flocculation is needed.
For instance, an extremely deflocculated casting slip will take longer to cast and yield a “brittle” cast piece that cannot be easily fettled. On the other hand, it would be possible to quickly cast a flocculated slip, but such a slip will retain excess water in the cast, and yield a cast piece that may probably distort prior to drying.
Summary
Although zeta potential is a robust tool, it should be used along with rheological measurements. It should also be perceived as a research and development tool that produces results. These results can then be matched against the performance of an end product, as a means of boosting confidence in the use of simpler quality control tests.
When zeta potential is applied to an improvement goal or industrial issue, suspension experts should work closely with the industrialists as a team. Analytical work must be agreed upon and assessed by all parties to make sure that the end objective of the industrialist is achieved.