Please note: This product is for research use only.
High Throughput NMR for Development and Validation of High-Quality and Cost-Effective IVD-by-NMR research and pre-clinical in vitro Screening Assays
Standardized nuclear magnetic resonance (NMR) spectroscopy platform enabling cost-effective, high-performance NMR pre-clinical screening and IVD-by-NMR discovery and validation (on RUO Level) of novel NMR assays. The new AVANCE IVDr system, presently for research use only, is a complete, proven and standardized platform for NMR pre-clinical research and screening, as well as for IVD-by-NMR research.
It features high sensitivity and information-rich output at 600 MHz proton-NMR frequency, and incorporates advanced hardware, software, automation, spectral libraries and standard operating procedures (SOPs) for high-performance biofluid assay validation and pre-clinical screening. Customer benefits include higher information content and spectral feature differentiation compared to low-field NMR systems, as well as excellent reproducibility, high throughput and potentially dramatically lower cost per sample for better preparation and support of clinical screening and IVD-by-NMR discovery and validation (on RUO level).
High Performance and Throughput IVD-by-NMR Research
Dedicated to NMR-based pre-clinical in vitro screening and research, the AVANCE IVDr is optimized for ease-of-use and highest data quality, reliability and reproducibility as shown in the figure on the right side.
The new standardized platform offers barcode analysis, control by a LIMS system, the high-throughput autosampler SampleJet™, remote access, and automatic analysis and customizable analytical result reporting.
Based on Bruker-validated SOPs, the AVANCE IVDr platform enables the development of future potential diagnostic tools for body-fluids that can address a variety of medical questions.
The SOPs guarantee the preparation of highly reproducible clinical data, enabling the exchange and validation of novel NMR assays between laboratories on a global basis. In a translational clinical research environment the results produced by these NMR assays can easily be transferred into potential clinical screening and future IVD use.
This level of large-scale health-related NMR screening is paving the way for worldwide epidemiological studies as well as for pre-clinical in vitro research. The benefits have been significant: facilitated by the low cost per sample and the even lower cost per parameter as compared to established single parameter screening methods.
Novel NMR methods for determining the cause of disease, delivering individualized patient treatment are now enabling many clinical researchers for developing strategies for prevention.
Reproducibility performance unmatched by Non-NMR methods
Overlay of 6 spectra each prepared and measured independantly on aliquots of a serum sample at Bruker BioSpin Rheinstetten and at Leiden University Medical Center in Holland, showing absolute identity of all spectra. Image Credit: Bruker BioSpin Group
Bruker standard operation procedures
Metabolite Reference Database
The BBIOREFCODE 2 is a database containing currently spectra of 800 compounds which are typically found as metabolites in body fluids or as components in samples from food, feed and beverage analysis. In addition also a variety of typical contaminants is contained.
AMIX Software provides powerful search algorithms to identify the compounds in the database in mixture spectra. This allows the assignment of unexpected signals in spectra from screening applications and the identification of the components.
Spectra are supplied at different pH values so that experimental conditions of mixture spectra and database can be matched for database search.
High Data Quality
All spectra supplied in the database are acquired at 600 MHz in aqueous solution under well defined conditions. Following the SOPs as for example used on the IVDr. The data is provided at up to 11 different pH values which allows a precise matching of the experimental conditions of the mixture spectra.
All spectra are cleaned-up (reviewable in history file, where every working step is listed) and fully assigned. They are stored in a special compressed format which reduces data volume dramatically and allows extremely fast search processes are well as simultaneous handling of large number of spectra.
Features
The NMR Data are acquired at 600MHz under well defined conditions. Proton Spectra are supplied at eleven different pH values from pH 3 to 8. For major pH 3, 5 and 7 2D spectra (JRES, COSY, TOCSY, HSQC and HMBC) are provided as well as 13C spectra for pH 3 and 7. All compounds are supplied with 3D-structure and the spectra are fully assigned. The data generation is a continuously on-going process. Once a database is installed it can be upgraded and extended with packages of new available compounds.
New
In June 2018 for the database for all pH-values BBIOREFCODE 2 a package of 50 new compounds was released. This database is now available with a total of 800 compounds.
B.I.-Methods
Clinical and translational research by NMR israpidly growing and worldwide networks of research groups such as the Phenome Center Network are forming. As a result, there is an increasing focus on standardization to maintain the transferability and reproducibility of the data. Currently there is an emphasis to standardize the following:
- NMR instrumentation
- Standard operation procedures
- Analysis procedures
- Metadata handling
Now available on the AVANCE IVDr system, B.I.-Methods is the basis for data analysis technology developments and enables standardization of body fluid analysis by NMR.
B.I.-Methods link the IVDr platform with Bruker's automatic data analysis service and contains all automation methods needed for standardized data generation of body fluids. It also includes efficient quality control methods needed when working on human samples and ensures transferability of spectral data generated. While the entire system is for research use only, B.I.-Methods paves the way for possible future use in clinical settings by fulfilling certain requirements for accredition and certification.
B.I.-Methods Includes
- Standard operating procedures for sample preparation
- Standardized parameter sets for body fluid and quality control measurements
- Quality control reporting system including validation reports and control charts
- QuantRefManager fully integrated in TopSpin to manage quantitative calibration of the IVDr system for urine, plasma/serum and CSF
- Automatic configuration of IconNMR according to the requirements of the IVDr system
- Standardized templates for SampleTrack
- Module for automatic access to Bruker Data Analysis Server
- IVDr Data Browser allows an easy management of the IVDr data (spectra and metadata) and enables to select, copy or overlay a part of them
Benefits of B.I.-Methods
- Standardized data generation and high data quality obtained in full automation
- Stringent spectrometer calibration and QC validation including stringent reporting„„
- Enabling pooling of data acquired at multiple systems into joint databases„„
- Enabling sharing and co-development of statistical models across laboratories
- Reliable and consistent NMR data generation in large scale studies with long term horizons„„
- Supporting NMR laboratory network approach including phenome center networks

Image Credit: Bruker BioSpin Group
Long term reproducibility of plasma (upper plot) and urine spectra (lower plot) acquired using B.I. Methods. Individual samples were prepared and measured as monthly quality controls within the framework of a lab QC system.
Source: Bruker BioSpin Group

Table 1. Overview of body fluid NMR related parameter sets of B.I.-Methods. Source: Bruker BioSpin Group

Table 2. Overview of calibration and quality control (QC) methods, reporting and control charts included in B.I.-Methods.
Purchasing the B.I.-Methods Package
The B.I.-Methods package comes standard with the AVANCE IVDr by NMR system. It can also be ordered to upgrade suitable non-IVDr systems to IVDr or IVDr compatible systems. Installation of IVDr Methods is done by specially trained service engineers as part of the IVDr set up or as upgrade to non-IVDr
systems. This ensures the B.I.-Methods package is correctly installed and the instrument is properly calibrated and connected to Bruker Data Analysis Server.
Reproducible Metabolite Quantification in Urine
Urine metabolic analysis is particularly valuable and information-rich, because the metabolic pathways of a wide range of nutrients, drugs and environmental contaminants can be identified in urine, even if urine is very complex to analyze. Large sample cohorts in clinical studies demand automated solutions capable of measuring and analyzing with highest information content and reproducibility.
The new B.I.QUANT-UR™ module of Bruker’s successful Avance® IVDr (In Vitro Diagnostics research) platform now provides precise, sensitive and fully reproducible results that have demonstrated great potential in preclinical animal and clinical/translational research.
B.I.QUANT-UR is available in three versions
- ƒƒB.I.QUANT-UR b: basic version, 50 compounds with concentration ranges, occurring in most human urines
- B.I.QUANT-UR e: extended version, 150 compounds with concentration ranges, age 6 month and up, also includingIEM and other disease markers
- B.I.QUANT-UR ne: neonates extended version, 150 compounds with concentration range, also including disease markers and including non-targeted classification against healthy newborn model
Use B.I.QUANT-UR to further your studies in:
- Epidemiology
- Frequent diseases like kidney damage, diabetes, metabolic syndrome, obesity and cancer
- Ability to monitor and optimize treatment
- Microbiom related health problems
- Food and environmental influence to health
- Monitor compound concentrations in personalized urinary metabolic profile
The extended versions can be used in those applications plus:
- Pediatrics
- Drug efficiency and treatment monitoring for IEM patients
- Functional food efficiency and dosage/composition
- Selective screening
- Neonatal health
Standardized Quality Control
While the number of Biobanks worldwide is growing rapidly, quality control of the whole process Is a requirement to ensure the value of the biobanks. Standardization is needed to allow researchers to integrate results obtained of specimen tests from 1 or more biobanks.
Standardization also includes the QC Process, which needs to cover all aspects of pre-analytics and sample storage. In addi- tion validation of specimen/donor metaparameters is of addi- tional value. NMR is especially suited to perform QC-analysis of liquid biopsies and can deliver a large number of criteria based on one QC measurement per sample.
In addition to QC Information NMR can deliver a large number of metabolic information using the same spectra generated during the QC process. With this information in urine 150 metabolites in 2 age ranges are quantified. In plasma/serum 115 lipoprotein related parameters (including subclasses) and 26 metabolites/parameters are analyzed and quantified, the whole process is under push button automation and can be handled by a trained medical technical assistant.
Example BioBank QC Summary
Extract of the summary page of the B.I.BioBankQC-PS and B.I.BioBankQC-UR report.
Image Credit: Bruker BioSpin Group
IVDR Platform and Its Embedded Solutions
Image Credit: Bruker BioSpin Group
Rich Spectral Information
Based on the outstanding performance of NMR in reproducibility and transferability, high quality analysis of data is available to deliver additional information for the biobank specimens. Together with the biobank, tool packages are offered for plasma/serum and urine quantification as outlined before. A multitude of disease relevant as well as endog- enous metabolites and lipoprotein information is delivered extracted from the QC spectra analysis.
Standardized Reporting

Image Credit: Bruker BioSpin Group
Example of the QC report available from B.I.BioBankTool
Spectra Instead of Aliquots
Based on the IVDr Platform concept and its strict standardiza- tion for NMR data generation, it is possible to select spectra from multiple biobanks for large epidemiological studies on a worldwide basis or to expand the testing range of clinical trials, providing for example spectra from healthy cohorts out of the biobanks Instead of generating always new aliquots. This builds a new value proposition for biobanks, allowing to save cost and contribute to big data in an efficient way. New NMR based diagnostic tests can such be validated on a worldwide basis and multiple phenotypes without exploding the cost of the trial. Data generated from a 11 IVDr platform ringtest clearly proof this unique feature of NMR.
Reproducible Metabolite Quantification in Plasma/Serum
The Bruker IVDr platform is optimized towards supporting the analysis of epidemiological studies with large scale, dedicated clinical and translational trials. The use of the standardized Bruker IVDr Methods module (B.I.Methods) guarantees high reproducibility and data quality under full automation indepen- dant from the NMR system and operator.
The Bruker IVDr platform allows to combine different solutions. Thus following our already established Bruker IVDr Lipoprotein Subclass Analysis (B.I.LISA TM) in plasma/serum samples, we now expand our biomarker panel with the simultaneous quantification of small molecules using the same spectrum as acquired for B.I.LISA. The automated quantification in plasma/serum (B.I.QUANT-PS TM) is based on in house developed algorithms involving fitting pre- defined 1H signals as it is already the case for urine samples (B.I.QUANT-UR TM).
Benefits of B.I.QUANT-PS
- Ease of use, simple and rapid sample preparation
- Fully automated quantification of 26 metabolites
- Different analytes classes can be quantified simultaneously in a single run
- Absolute concentration for each metabolites are given due to the calibration with one Quantification Reference Sample (B.I.MethodsTM)
- Validation of all LODs has been done following ISO 17025 guidelines for wet spiking
- Rapid analysis: on a daily basis up to 160 samples can be prepared, measured and analyzed
- Retrospective analysis possible if the sample was prepared and measured using B.I.Methods module
- Works on plasma or serum samples
Example of Automated Report

Image Credit: Bruker BioSpin Group
Extract of B.I.QUANT-PS of a serum sample from a patient with high glucose.
Validation of quantification results
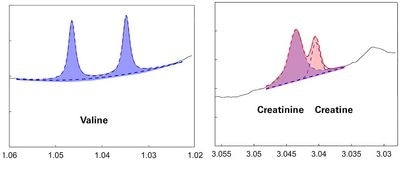
Image Credit: Bruker BioSpin Group
Further Information
The B.I.QUANT-PS module is offered at an annual flat rate. It is for research use only and not released for clinical diagnostics.