Track particles in real time to monitor and improve processes.
ReactIR for In Situ Reaction Analysis
The ReactIR FTIR spectrometer from METTLER TOLEDO has been designed for in situ reaction analysis. It allows scalable, stable, and reliable process development.
The ReactIR allows researchers to quantify the reaction trends and profiles in real-time, offering highly specific data about pathways, mechanisms, kinetics, and the effect of reaction variables on performance.
With the help of the ReactIR system, users can directly monitor reagents, reactants, products, by-products, and intermediates as they vary during the course of the reaction. The ReactIR offers crucial data to researchers as they explore, develop, and improve chemical processes, synthetic routes, and chemical compounds.
Reaction Analysis is Simplified
To gain a better understanding of chemical reactions, chemists should deal with the following:
- What are the reaction mechanism and kinetics?
- When does the reaction begin and when does it end?
- What happens when reaction temperature, mixing rates and dosing rates change?
- What is the impact of those transient intermediates?
- Did it react as anticipated, and did any by-products develop and why?
To achieve the most optimal data and inspect the reactions rapidly, the ReactIR FTIR spectrometer leverages five areas so that reaction understanding is easily available to all the chemists—expert or novice.
In-Situ Fourier Transform Infrared (FTIR) Spectroscopy with ReactIR
Video Credit: METTLER TOLEDO
Best-in-Class Performance
Whether it is a detector, probe, or software, the ReactIR has been improved for use in the mid-IR “fingerprint” region—leading to an extremely sensitive system for precise and rapid molecular data.
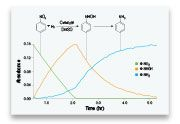
Image Credit: METTLER TOLEDO
One-Click Analytics
Particularly developed for time-resolved reaction analysis, the iC IR software integrates a peak picking algorithm along with functional group intelligence to considerably decrease the analysis time. Users can integrate their chemistry knowledge with an automated data analysis workflow to guarantee proper collection and understanding for each experiment.

Image Credit: METTLER TOLEDO
Broad Range of In Situ Probes
Probes developed to work from low-to-high pressure, low-to-high temperature, and under basic, acidic, caustic, aqueous, and oxidizing conditions allow the analysis of almost all types of chemistries.

Image Credit: METTLER TOLEDO
Solutions from Laboratory to Plant
The ReactIR FTIR spectrometers are compact enough to be accommodated inside a fume hood, are ATEX-rated to deploy in a plant, and feature sampling technology to sample any kind of process or reaction. They can be used to demonstrate that what takes place in the plant is what users witnessed in the laboratory.

Image Credit: METTLER TOLEDO
Extensive Reaction Analysis Experience
As a company, METTLER TOLEDO has more than three decades of dedicated reaction analysis experience. This is its passion and focus. The company has built this expertise into FTIR spectrometers that are fit for purpose.

Image Credit: METTLER TOLEDO
ReactIR in Recent Journal Publications
Constant measurements from infrared spectrometers are used to acquire reaction profiles to estimate the reaction rates. A publication list from peer-reviewed journals targets new and interesting applications of the ReactIR spectrometer. Scientists in both industry and academia use in situ mid-FTIR spectrometers to get rich experimental data and in-depth information to improve their analyses.
Featured ReactIR citations
- Drew Hood, Ryan Johnson, Alex Carpenter, Jarod Younker, David Vinyard, and George Stanley. “Highly active cationic cobalt(II) hydroformylation catalysts.” Science. January 31st, 2020: Volume 367, Issue 6477, pp. 542-548.
- B. Wei, J.C Sharland, P. Lin, S.M Wilkerson-Hill, F. A. Fullilove, S. Mckinnon, D.G Blackmond, and H. M. L. Davies. “Low Catalyst loading: Rhodium (II)-Catalyzed Asymmetric Cyclopropanation.” ACS Catalysis. 2020, 10, 1161–1170.
- Xuhui Chen, ab, Derek B. Rice, ac, Andrew M. Danby, ab, Michael D. Lundin, ab, Timothy A. Jackson, ac, and Bala Subramaniam. “Experimental and computational investigations of C–H activation of cyclohexane by ozone in liquid CO2 React.” Chemical Engineering. 2020, 5, 793.
- Mei Carmen, Deshmukh Sasmit, Cronin James (et al), “Aluminum Phosphare Vaccine Adjuvant: Analysis of Composition and size using off-line and in-line Tools.” Computational and Structural Biotechnology journal (2019) volume 17 pp1184-1194.
- Meng Shan-Shui, Lin Li-Rong, Luo Xiang, Lv Hao-Jun, Zhao Jun-Ling, and Chan Albert S. C., “Aerobic oxidation of alcohols with air catalyzed by decacarbonyldimanganese” (2019) Green Chemistry issue 22.
Why Select ReactIR over Offline Analysis?
Conventionally, to achieve reaction data, HPLC is used to collect samples for offline analysis. For chemistries where the removal of samples leads to loss of crucial data, or are dangerous or otherwise toxic, this is not a straightforward procedure. Chemists also need to be present to collect the sample and subsequently wait for the results before the initiation of reaction analysis.
These issues have the following implications:
- Damage to the intermediate results in inaccurate pathway hypothesis
- The sample may not be representative
- Poor knowledge of pressurized, explosive, toxic, and air-sensitive systems
- Crucial events that affect the quality of a product or process may be overlooked
- Incorrect data leads to longer development times because of the change in reaction
Reaction Analysis Case Study: Transient Intermediate Detection #TCFH
Video Credit: METTLER TOLEDO
ReactIR is Ready!
The ReactIR 702L is the world’s first system that actually combines the power of in situ, real-time FTIR with corresponding operational convenience. The ReactIR is ready for every experiment and for every chemist.
ReactIR is Ready to Run Overnight!
The ReactIR 702L employs solid-state cooling technology to offer the best-in-class performance, without the need for liquid nitrogen. By avoiding the need for repetitive Dewar refills and hazardous setup, researchers can effortlessly track the chemistry over an extended period of time.
Image Credit: METTLER TOLEDO
ReactIR is Ready to Grab and Go!
Stackable and compact units save valuable space in the fume hood, providing flexibility to install the ReactIR spectrometer in different locations over the laboratory. An “always on” detector decreases the set-up time and allows researchers to begin their data collection with confidence at a moment’s notice.
ReactIR is Ready for Users’ Chemistry!
Through probe- and flow-based sampling technologies, researchers can analyze gas and liquid phase chemistry in continuous setups or batch setups. Fit-for-purpose construction materials make data collection easy and simple in corrosive and acidic settings over a broad range of pressures and temperatures.
ReactIR Applications
The ReactIR spectrometer operates in a broad range of chemistries, where the chemistry is off-gas or in solution, the molecule is infrared active, and the concentration is greater than about 0.1 %.
ReactIR: FTIR Spectrometer Technology & Applications
Video Credit: METTLER TOLEDO
Standard application areas include:
- Hydrogenation reactions
- High-pressure reactions
- Polymerization reactions
- Halogenation
- Grignard reaction mechanisms
- Hydroformylation or oxo synthesis/process
- FTIR spectroscopy
- Catalyzed reactions
- Flow chemistry
- Biocatalysis/enzymatic catalysis
ReactIR vs Raman—A Comparison
Raman and ReactIR spectrometers are usually interchangeable and provide complementary data, but there are practical variations that influence which system is optimal. Most molecular symmetry will enable both Raman and FTIR activity. In a molecule containing a center of inversion, Raman bands and IR bands are equally exclusive (that is, the bond will either be IR active or Raman active but not both).
One basic rule is that functional groups with major changes in dipoles are powerful in the IR, while functional groups with a high degree of symmetry or with weak dipole changes and no net dipole change will be better observed in Raman spectra.

Image Credit: METTLER TOLEDO
The ReactIR can be selected when:
- Bonds with powerful dipole changes are significant, for example, N=O, O-H, and C=O
- Reactions in which the solvents, reagents, reactants, and reaction species fluoresce
- Reactions in which the solvent bands are powerful in Raman and can swamp the signal of important species
- Reactions where the resultant intermediates are IR active
- Reactions where there is a low concentration of reagents and reactants
Raman can be selected when:
- Analysis of particles in solution is significant (for example, polymorphism)
- Bonds that are difficult to observe in FTIR (for example, S-H, 0-0, C=C, C=S, N=N, and so on)
- Analyzing carbon bonds in aromatic and aliphatic rings are of major interest
- Analyzing reactions in aqueous media
- Lower frequency modes are significant (for example, metal-oxygen)
- Analysis of reaction initiation, endpoint, as well as product stability of colloidal and biphasic reactions
- Analysis of lower frequency lattice modes is important
- Reactions where observation via a reaction window is both safer and easier (for example, polymerizations and high-pressure catalytic reactions)
What is Hiding Between the HPLC Samples?
Here, five examples collected from new journal articles are presented. In these articles, the ReactIR achieves tasks that would be impossible, challenging, or overly time-intensive, if performed with conventional offline methods:
- Track difficult-to-sample chemistry—lithiation reaction can be carried out at −70 °C
- Expose reaction mechanisms—detection of transient intermediates in a coupling reagent
- Prevent hold time to boost throughput and quality—decomposition leading to epimerization
- Track reaction progress for improved purity and yield—determination of optimal reaction endpoint
- Quickly determine kinetics—first-order reaction kinetics in a single experiment
Image Credit: METTLER TOLEDO