Unattended, representative sampling for a wide range of chemical reactions.
Some Reactions are Difficult to Sample Without Affecting Reaction Progression
It is almost impossible to reliably take representative samples of oxygen or moisture-sensitive reactions and reactions at very high pressure or sub-ambient temperatures. Extremely toxic reactions pose further challenges. The innovative patented EasySampler probe allows the capture and instant quenching of reactions to offer a sample representative of a reaction during sampling.
EasySampler - Automated Sampling System for Chemical Reactions
Video Credit: METTLER TOLEDO
Sample and Quench at Reaction Conditions
How Does it Work?
The EasySampler is submerged into a reaction, where it stays for the entire period of the experiment. The sample pocket moves out and a sample is filled into it. When the pocket withdraws, the sample is quenched instantly, at reaction conditions. Then, the sample is diluted to a user-defined concentration and ready for offline examination.
How EasySampler Takes Samples
Video Credit: METTLER TOLEDO
Representative and Reproducible Samples
From Heterogeneous and Multiphase Reactions
Sampling reactions with precision is a tough task. Sampling is particularly hard for heterogeneous and multiphase reactions. The EasySampler carries out sampling from the same point in the reactor, into a pocket of fixed volume and contains solids.
Dissolution of solids starts instantly, with the quench and dilution steps, thus supplying dissolved samples to gain representative, reproducible, and correct analytical data.

Image Credit: METTLER TOLEDO
Automated and Unattended 24/7
Never Miss a Sample Again
Sampling lethal or long reactions, or reactions for Design of Experiments (DoE) studies, can be laborious and almost impossible, resulting in blind spots in data. Thus there is often a need to repeat experiments. The EasySampler samples the same way each time and can be configured for nonstop sampling operations 24/7 to offer data sets for full reaction understanding, thus enhancing productivity.
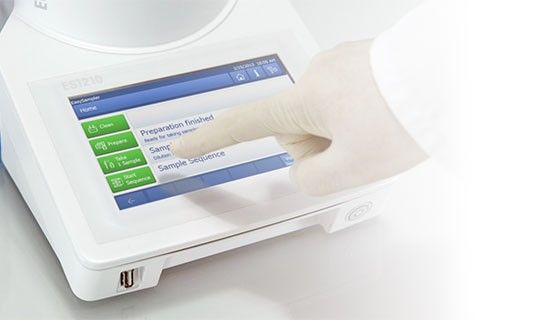
Image Credit: METTLER TOLEDO
Sample Preparation
For HPLC-Ready Concentrations
Following the quenching process, the liquid handling system dilutes the sample to a user-defined concentration and conveys the sample to a vial, delivering it ready for offline examination. It is possible to use these steps for derivatization, thereby reducing sample preparation time and preventing human error.
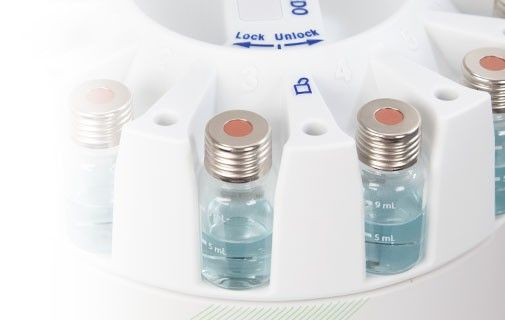
Image Credit: METTLER TOLEDO
Eliminate User Safety Concerns
Related to Toxic and Hazardous Chemistries
The EasySampler makes the sampling process completely automatic, preventing the need for manual handling of liquids. This decreases risks and enhances operator safety, particularly with:
- Hazardous reactions at very high temperatures
- Extremely toxic chemistry
- Other risky reaction conditions

Image Credit: METTLER TOLEDO
Faster and Improved Chemical Development
With Integrated Reactor Systems and EasySampler

Image Credit: METTLER TOLEDO
Plug-and-play combination of OptiMax, EasyMax Advanced, and RX-10 with EasySampler allows faster chemical development. Automated reactors regulate reaction parameters while performing experiments 24 hours per day.
Incorporating the EasySampler offers representative samples during the course of the reaction and superior quality HPLC data for comprehensive and accurate reaction profiles. The iControl software regulates the reactor system and EasySampler, and reports all experiment-related data in a single file.
These integrated instruments can be used by researchers to swiftly comprehend, innovate, and make informed decisions so that reactions can be enhanced in a shorter span of time.
Common Applications of the EasySampler
- Conversion
- Yield
- Reaction kinetics
- Impurity profiling
- By-product formation
- Air-sensitive reactions
- Design of experiment studies
- Moisture-sensitive reactions
- Reactions at elevated pressure
- Hazardous and toxic reactions
- Reactions at sub-ambient temperatures
- Homogeneous reactions

Image Credit: METTLER TOLEDO