May 1 2008
Researchers at the National Institute of Standards and Technology (NIST) and Max Planck Institute for Physics in Germany believe they can achieve a significant increase in the accuracy of one of the fundamental constants of nature by boosting an electron to an orbit as far as possible from the atomic nucleus that binds it.
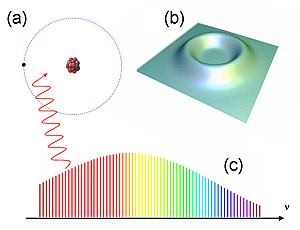 (a) In a Rydberg atom, an electron (black dot) is far away from the atomic nucleus (red and grey core). (b) Probability map for an electron in a Rydberg atom shows that it has virtually no probability of being near the nucleus in the center. (c) An optical frequency comb for producing ultraprecise colors of light can trigger quantum energy jumps useful for accurately measuring the Rydberg constant.
(a) In a Rydberg atom, an electron (black dot) is far away from the atomic nucleus (red and grey core). (b) Probability map for an electron in a Rydberg atom shows that it has virtually no probability of being near the nucleus in the center. (c) An optical frequency comb for producing ultraprecise colors of light can trigger quantum energy jumps useful for accurately measuring the Rydberg constant.
The experiment, outlined in a new paper,* would not only mean more accurate identifications of elements in everything from stars to environmental pollutants but also could put the modern theory of the atom to the most stringent tests yet.
The physicists’ quarry is the Rydberg constant, the quantity that specifies the precise color of light that is emitted when an electron jumps from one energy level to another in an atom. The current value of the Rydberg constant comes from comparing theory and experiment for 23 different kinds of energy jumps in hydrogen and deuterium atoms. Researchers have experimentally measured the frequencies of light emitted by these atomic transitions (energy jumps) to an accuracy of as high as 14 parts per quadrillion (one followed by 15 zeros), but the value of the Rydberg constant is known only to about 6.6 parts in a trillion—500 times less accurate. The main hurdle to a more accurate value comes from uncertainties in the size of the atom’s nucleus, which can alter the electron’s energy levels and therefore modify the frequency of light it emits. Another source of uncertainty comes from the fact that electrons sometimes emit and reabsorb short-lived “virtual photons,” a process that also can slightly change the electron’s energy level.
To beat these problems, NIST physicist Peter Mohr and his colleagues propose engineering so-called hydrogen-like Rydberg atoms—atomic nuclei stripped of all but a single electron in a high-lying energy level far away from the nucleus. In such atoms, the electron is so far away from the nucleus that the latter’s size is negligible, and the electron would accelerate less in its high-flung orbit, reducing the effects of “virtual photons” it emits. These simplifications allow theoretical uncertainties to be as small as tens of parts in a quintillion (one followed by 18 zeros).
NIST researchers Joseph Tan and colleagues hope to implement this approach experimentally in their Electron Beam Ion Trap Facility. The idea would be to strip an atom of all its electrons, cool it and inject a single electron in a high-flying orbit. Then the researchers would use a sensitive measurement device known as a frequency comb to measure the light absorbed by this Rydberg atom. The result could be an ultraprecise frequency measurement that would yield an improved value for the Rydberg constant. Such a measurement would be so sensitive that it could reveal anomalies in quantum electrodynamics, the modern theory of the atom.
Posted May 1st,2008