Jul 2 2009
Carbon dioxide contributes to the greenhouse effect, but practicable solutions for its capture and storage have not really been found. However, it might be possible to kill two birds with one stone if carbon dioxide could be used as a raw material. Unlike the carbon sources commonly used today-fossil fuels and natural gas- carbon dioxide is a renewable resource and an environmentally friendly chemical reagent. Unfortunately, the carbon-oxygen bonds are too strong to be broken easily. Until now, this has mainly been achieved with the use of metal-containing catalysts. A German-Canadian cooperative effort has now developed a new concept that works without metals: as they report in the journal Angewandte Chemie, the team led by Gerhard Erker and Stefan Grimme at the University of Münster and Douglas W. Stephan at the University of Toronto uses so-called frustrated Lewis acid/base pairs to reversibly bind carbon dioxide under mild conditions.
 © Wiley-VCH
© Wiley-VCH
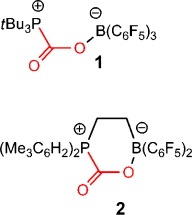
An organic borane and an organic phosphine form a typical Lewis pair: As a Lewis base, the phosphine has too many electrons. The borane on the other hand, a Lewis acid, has an electron deficiency. The Lewis base thus makes its free electron pair available to the Lewis acid. The phosphine and borane form an adduct that is held together by way of the shared electron pair. However, if both partners have bulky side groups, they cannot come together to form the desired bond. They are then described as a “frustrated” Lewis pair.
The researchers exposed a solution of such a frustrated pair to an atmosphere of CO2 under two bars of pressure. This immediately resulted in a reaction, which formed a white solid. What happened? The phosphorus atom in the frustrated phosphine uses its electron pair to bind to the carbon of the CO2, and the boron atom of the frustrated borane snaps up the free electron pair of one of the oxygen atoms of the CO2 and binds to it. In this way, the carbon dioxide couples the two partners together, alleviating their frustration.
With the application of heat or certain solvents, the carbon dioxide can be released and the Lewis pair returned to its original frustrated state. The researchers are now investigating how the captured CO2 could be chemically transformed for use as a feedstock.