Apr 16 2015
A team of researchers at DESY's X-ray source PETRA III, has observed structural changes in lithium-ion batteries for the first time. Led by Dr. Ulrike Bösenberg, the researchers performed fluorescence studies, which revealed that the internal structure of the battery material was significantly damaged following just a few charging cycles.
 Researchers have observed structural changes in lithium-ion batteries for the first time. They performed fluorescence studies, which revealed that the internal structure of the battery material was significantly damaged following just a few charging cycles.
Researchers have observed structural changes in lithium-ion batteries for the first time. They performed fluorescence studies, which revealed that the internal structure of the battery material was significantly damaged following just a few charging cycles.
When lithium-ion batteries are charged rapidly, it can reduce their capacity permanently. This also causes deactivation of the areas of the energy storage structure. However, such degrees of destruction do not occur immediately when charging is done slowly.
Generally, lithium-ion batteries have a high charge density, but when they are charged and discharged several times, their storage capacity is considerably reduced. Lithium-nickel-manganese-oxide spinel materials, also known as LNMO spinels, offer a potential solution to this issue. LNMO spinels have a high voltage of 4.7V, which make them a viable option for next-generation energy storage systems. The electrodes of these spinels include small crystals, or crystallites. These crystals are in turn linked to the conductive carbon and the binder material to create a thin layer.
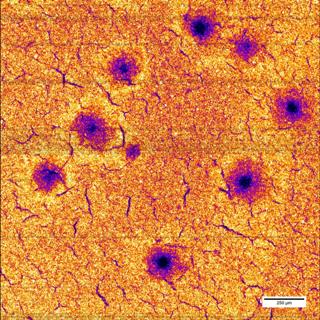
Other researchers who took part in this study were from the University of Hamburg, University of Giessen, and Australia's national science agency CSIRO. The group used PETRA III's X-ray microfocus beamline P06 to analyze the negative electrodes of the iNi0.5Mn1.5O4 compound. With just half a micrometer resolution, the researchers were able to determine the actual distribution of manganese and nickel across wide areas of the electrode by means of an innovative X-ray fluorescence detector.
The battery electrodes include an active material, which molecular structure contains oxygen, manganese, and nickel. This structure serves as a stiff crystal lattice and makes it possible to extract or insert the lithium ions.
During the analysis, each battery electrode was subjected to 25 charging and discharging cycles at three varied rates and the elemental distribution of the electrode components was subsequently determined. It was observed that during rapid charging, both nickel and manganese atoms are leached from the crystal lattice. In addition, some defects were also observed, such as holes in the electrode which measured 0.1mm in diameter. The areas subsequently destroyed could not be used for lithium storage.
For each chemical element, using XRF, the energy or wavelength of the fluorescent radiation represents a typical fingerprint. In this manner, it was possible to ascertain the distribution of separate materials in the electrode.
To achieve this outcome, an innovative fluorescence detector called a Maia detector was used. This instrument was jointly developed by Brookhaven National Laboratory and CSIRO in the US and contains 400 individual elements which are capable of collecting the fluorescent radiation from the sample. Thanks to its high sensitivity and excellent energy resolution, the detector was able to localize a number of chemical elements at the same time.
Additionally, the high and narrow-intensity PETRA III X-ray beam was able to scan the surface of the sample accurately which measured 2x2mm2, with half a micrometre resolution. This means, each point could be analyzed rapidly.
However, researchers are still not clear where the dissolved manganese and nickel atoms end up and hope to address this question in their future studies.
It is the first time that we could localize these inhomogeneities with such a high spatial resolution over so large an area. We hope to better understand the effects and to create the foundation for improved energy storage devices. There are indications that the dissolved material, at least partially, settles on the anode, which inflicts twice the damage to the battery properties.
Dr. Ulrike Bösenberg
The study results will appear in the latest edition of the academic journal Chemistry of Materials.