Aug 17 2016
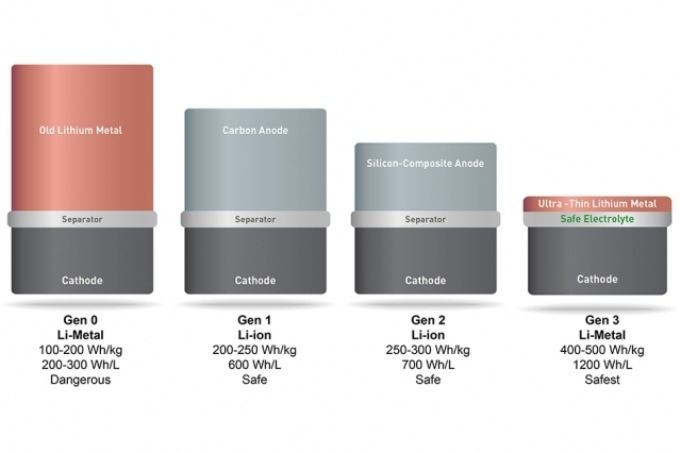 SolidEnergy Systems' battery (far right) is twice as energy-dense, yet just as safe and long-lasting as the lithium ion batteries used in consumer electronics. The battery uses a lithium metal foil for an anode, which can hold more ions and is several times thinner and lighter than traditional lithium metal, graphite, carbon, or silicon anodes. A novel electrolyte also keeps the battery from heating up and catching fire. (Credit: SolidEnergy Systems)
SolidEnergy Systems' battery (far right) is twice as energy-dense, yet just as safe and long-lasting as the lithium ion batteries used in consumer electronics. The battery uses a lithium metal foil for an anode, which can hold more ions and is several times thinner and lighter than traditional lithium metal, graphite, carbon, or silicon anodes. A novel electrolyte also keeps the battery from heating up and catching fire. (Credit: SolidEnergy Systems)
A novel rechargeable lithium metal battery, capable of providing double the energy as other lithium ion batteries used in consumer electronics today will be entering the commercial market, thanks to an MIT spinout.
SolidEnergy Systems was established in 2012 by MIT alumnus and former postdoc Qichao Hu ’07. The company has created an “anode free” lithium metal battery with numerous advances in the materials that give it the same longevity and make it just as safe as the lithium ion batteries used in electric cars, drones, wearable electronics, smartphones and other devices, while being twice as energy-dense.
With two-times the energy density, we can make a battery half the size, but that still lasts the same amount of time, as a lithium ion battery. Or we can make a battery the same size as a lithium ion battery, but now it will last twice as long.
Qichao Hu, CEO of SolidEnergy
The battery replaces graphite, a material commonly used as battery anode, with high energy, extremely thin lithium-metal foil. The foil can contain more ions and increases the energy capacity.
Chemical alterations in the electrolyte make the often volatile and short-lived lithium metal batteries safe and rechargeable. In addition, the batteries can be manufactured with the existing equipment used for lithium ion production, which accounts for scalability.
SolidEnergy presented the first working prototype of a rechargeable lithium metal smartphone battery with twice the energy density, in October 2015; an endeavor through which the company gained over $12 million from its investors.
The battery offers 2.0 amp hours as compared to the lithium ion battery that offers 1.8 amp hours, and at half the size of an iPhone 6 lithium ion battery.
The company intends to introduce the batteries in smartphones and wearables at the beginning of 2017 and in electric cars by 2018. The first application, however, will happen in November 2016, with drones.
Several customers are using drones and balloons to provide free Internet to the developing world, and to survey for disaster relief. It’s a very exciting and noble application. [Introducing these lithium metal batteries in electric vehicles as well could stand for] a huge societal impact. Industry standard is that electric vehicles need to go at least 200 miles on a single charge. We can make the battery half the size and half the weight, and it will travel the same distance, or we can make it the same size and same weight, and now it will go 400 miles on a single charge.
Qichao Hu, CEO of SolidEnergy
Tweaking the “Holy Grail” of Batteries
Scientists have endeavored in vain for decades to develop rechargeable lithium metal batteries as they provide increased energy capacity. “It is kind of the holy grail for batteries,” Hu says.
One setback is the poor reaction that lithium metal has with the electrolyte, a solution that conducts ions between the positive and negative electrodes, cathode and anode, in the battery. The unfortunate reaction results in the formation of compounds that lead to shorter battery life and more battery resistance.
Another unfavorable reaction is the creation of dendrites or mossy lithium metal bumps that create short circuits. The high levels of heat generated lights the inflammable electrolyte which makes the batteries nonrechargable.
In order to make the batteries safer, the performance of the battery has to be compromised by substituting liquid electrolyte with inorganic electrolyte that is hard to scale up or with solid polymer electrolyte that is not as conductive and that require high temperature heating for operation.
Hu, a popular battery researcher who has created many liquid metal and molten salt batteries, contributed a lot in making numerous material advancements and key designs while he was a part of MIT professor Donald Sadoway’s group. These designs and advancements form the basis of SolidEnery’s technology.
One such advancement was introducing ultrathin lithium metal foil as the anode. The foil is a lot lighter and thinner than the conventional carbon, silicon or graphite anode and only one-fifth the thickness of conventional lithium metal anode. This reduces the size of the battery by half.
However, there was yet another serious drawback as the battery would function only at a temperature of 80°C or more.
“That was a showstopper,” Hu says. “If the battery doesn’t work at room temperature, then the commercial applications are limited.”
To tackle these drawbacks, Hu created a liquid and solid hybrid electrolyte solution. He gave the lithium metal foil a coating of thin solid electrolyte that does not require heating in order to operate. He also developed a novel quasi-ionic liquid electrolyte that is not flammable and has extra chemical alterations to the cell design and separator in order to prevent negative reaction of the lithium metal with the electrolyte.
The resulting battery was one with the long life and safety of lithium ion batteries that can function at room temperature and the increased energy-density of the lithium metal batteries.
Combining the solid coating and new high-efficiency ionic liquid materials was the basis for SolidEnergy on the technology side.
Qichao Hu, CEO of SolidEnergy
Blessing in Disguise
For the business part, Hu frequently visited the Martin Trust Center for MIT Entrepreneurship to get important guidelines from investors and mentors. He also joined the Course 15.366 (Energy Ventures), where he brought together a team to create a business plan for the new battery.
The business plan won the team the first prize at the MIT $100K Entrepreneurship Competition’s Accelerator Contest and took it to the finalists at the MIT Clean Energy Prize. The team then represented MIT and won the second prize at the national Clean Energy Prize competition that was held at the White House.
Hu was planning the launch of SolidEnergy in late 2012, when another MIT spinout, A123 Systems, which was known for creating lithium ion batteries, registered bankruptcy. The market did not seem favorable for battery companies. “I didn’t think my company was doomed, I just thought my company would never even get started,” Hu says.
However, this proved to be a blessing in disguise. Hu’s MIT connection allowed him to use A123’s facilities in Waltham, including manufacturing equipment and clean and dry rooms, which was not in use then, to prototype. After Wanxiang Group acquired A123 in 2013, SolidEnergy entered a collaboration agreement with the company to continue using the resources.
At the facility SolidEnergy was left with no choice but to create a prototype using existing lithium ion producing equipment. This led the company to design novel, yet commercially useable, batteries. Hu comments that battery companies that have new material advancements most often create new production processes according to the new materials that are not always scalable or practical. “But we were forced to use materials that can be implemented into the existing manufacturing line,” he says. “By starting with this real-world manufacturing perspective and building real-world batteries, we were able to understand what materials worked in those processes, and then work backwards to design new materials.”
SolidEnergy moved to its new modern pilot facility located in Woburn, after sharing its previous Waltham facility, that is ten times bigger and “can house the wings of a Boeing 747” Hu says. The company intends to increase the production for their launch in November.