Aug 31 2016
Merging two powerful 3-D X-ray techniques, a team of researchers from the Department of Energy's SLAC National Accelerator Laboratory and Utrecht University in the Netherlands revealed new details of a process known as metal poisoning that clogs the pores of catalyst particles used in gasoline production, causing them to lose effectiveness.
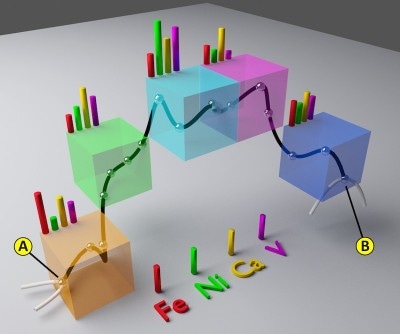 This illustration depicts concentrations of chemical elements at five different points in a catalyst pore channel. The zigzag represents the pore channel, which was reconstructed from X-ray microscopy imaging. The colors show the chemical composition, detected with X-ray fluorescence. This information was combined in a model that simulates the aging of the catalyst pore network.( Credit: SLAC National Accelerator Laboratory)
This illustration depicts concentrations of chemical elements at five different points in a catalyst pore channel. The zigzag represents the pore channel, which was reconstructed from X-ray microscopy imaging. The colors show the chemical composition, detected with X-ray fluorescence. This information was combined in a model that simulates the aging of the catalyst pore network.( Credit: SLAC National Accelerator Laboratory)
The team combined their data to produce a video that shows the chemistry of this aging process and takes the viewer on a virtual flight through the pores of a catalyst particle. The results were published today in Nature Communications.
The particles, known as fluid catalytic cracking or FCC particles, are used in oil refineries to “crack” large molecules that are left after distillation of crude oil into smaller molecules, such as gasoline. Those oil molecules flow through the catalyst particles in tiny pores and passageways, which ensure accessibility to the active domains where chemical reactions can take place. But while the catalyst material is not consumed in the reaction and in theory could be recycled indefinitely, the pores clog up and the particles slowly lose effectiveness. Worldwide, about 400 reactor systems refine oil into gasoline, accounting for about 40 to 50 percent of today’s gasoline production, and each system requires 10 to 40 tons of fresh FCC catalysts daily.
Finding new clues about how FCCs age out could be key to improving gasoline production. But the new technique also has potential for understanding the workings of materials for powering cars of the future, according to Yijin Liu, a lead author on the paper and staff scientist at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL), a DOE Office of Science User Facility.
"The model we created by combining these two imaging methods can readily be applied to studies of rapid changes in the pore networks of similarly structured materials, such as batteries, fuel cells and underground geological formations," he said.
Two Perspectives Complete the Picture
To design materials for tomorrow's energy solutions, scientists must understand how they work at multiple scales invisible to the human eye.
In a previous study at SSRL, the team took a series of two-dimensional images of catalyst particles at various angles and used software they developed to combine them into three-dimensional images of whole particles showing the distribution of elements in catalysts at various ages.
For the new study, the researchers examined an FCC particle recovered from a refinery using two different 3-D X-ray imaging techniques at two experimental stations, or beamlines, at SSRL.
One technique, called X-ray fluorescence, provided a detailed profile of the particle's chemical elements. The other, X-ray transmission microscopy, captured the nanoscale structure of the particle, including fine details about the porous network where metal poisoning can best be observed.
"The high-resolution microscopy data provided a map of the pores, and the high sensitivity of X-ray fluorescence showed us where metals in the refining fluids were poisoning the catalyst, which appeared as a colored fog in our visualization,” Liu said.
The results of the study highlight the importance of having multiple techniques to study a single sample at a facility like SSRL. "There was a lot of development on the beamlines to make it possible to register the data in 3-D at this very fine scale," Liu said. He heads up one of the two beamlines used in the research, which allows him to understand the strength and the limitations of both imaging methods.
“Understanding catalyst performance requires interrogating catalyst function from multiple perspectives," SSRL Director Kelly Gaffney said. "The results of this exciting research effort highlight the value of integrating disparate X-ray imaging methods to build a deeper understanding of materials function.”
A Model for Understanding Material Dynamics
Going beyond the observation of the experimental data visualized in the video, the scientists developed a model explaining how the accumulation of metals poisons the efficiency of the catalyst.
"We used an analogy between electrical resistance and the degree of pore blockage, between two points in the particle using the new combined data. We then applied formulas well-known in electrical engineering to explain accessibility through the pore network, but also how it changes when metals are blocking pores," said the study's co-lead researcher Florian Meirer, assistant professor of inorganic chemistry and catalysis at Utrecht University.
The resulting model simulates the aging of the catalyst, allows scientists to quantify this virtual aging, and helps them predict the collapse of its transportation network.
"The model explains for the first time how this happens in a connective manner, which is a big step toward improving the design of such catalysts. Furthermore, this novel approach can be applied to a broad range of other materials that involve the transport of fluids or gases, such as battery electrodes," said Bert Weckhuysen, professor of inorganic chemistry and catalysis at Utrecht University.
Other researchers who contributed to this work were SSRL's Courtney Krest and Samuel Webb. This work was supported by the NWO Gravitation program, Netherlands Center for Multiscale Catalytic Energy Conversion, and a European Research Council Advanced Grant.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science. For more information, please visit slac.stanford.edu.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time.