Mar 16 2017
Metal-based polymers have unique properties that make them suited for applications such as gels that change shape on oxidation or “self-healing” materials. Some of these properties arise from interactions between metal ions and ligands in the metallopolymers. The interactions normally involve one of two extremes: persistent covalent bonding, in which the polymers are stable, or labile coordination bonding, in which the polymer properties can be easily manipulated by solvent, heat, or light.
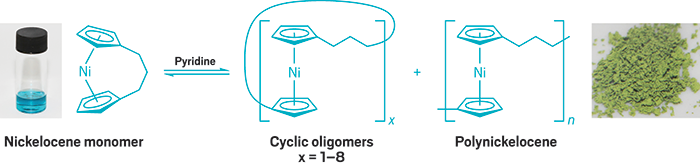 Adding a polar solvent such as pyridine pushes the equilibrium of this metallopolymer system to favor the nickelocene monomer. (Credit: Ian Manners)
Adding a polar solvent such as pyridine pushes the equilibrium of this metallopolymer system to favor the nickelocene monomer. (Credit: Ian Manners)
Rebecca A. Musgrave and Ian Manners of the University of Bristol and coworkers have now developed a new feature for metallocene-based polymers, showing that the molecules can switch between the stable and dynamic states. This ability could lead to new applications for stimulus-response materials and ease processing and recyclability of metallopolymers.
Polymers built from metallocene units such as ferrocenylsilane fall into the stable category because of the strong iron-cyclopentadienyl covalent bonds in the signature sandwich structures—the polymers are comparable in durability with polyethylene and polystyrene. On the other hand, polymers containing zinc coordination complexes have labile metal-ligand bonds and fall apart, for example when irradiated by light.
Manners’ team decided to take a look at crafting metallopolymers that could reversibly switch between stable and dynamic behaviors. This led them to nickelocene. Unlike ferrocene, which has 18 valence electrons, nickelocene has 20 valence electrons. The two extra electrons are unpaired and their presence leads to longer, weaker, and more easily cleaved metal-cyclopentadienyl bonds relative to ferrocene.
The researchers found they could use the bonding difference to control the material properties in the nickel-based polymer by choice of solvent, dilution level, temperature, and time. For example, polynickelocene remains stable in a low-polarity, noncoordinating solvent such as toluene, but undergoes dynamic depolymerization in a polar, coordinating solvent such as pyridine. In addition, the equilibrium favors the monomer if the solution is dilute, low-mass oligomers if moderately dilute, and polymer if concentrated (Nat. Chem. 2017, DOI: 10.1038/nchem.2743).
These switchable properties should add to the range of applications for metallopolymers, Manners says. In particular, the unpaired electrons in the nickel-based polymer offer the potential to create soluble and easily processed magnetic materials. Polyferrocenes, in contrast, lack interesting magnetic properties.
The new report is “groundbreaking,” says Chuanbing Tang of the University of South Carolina. “The labile chemical bond of nickelocene could open an avenue to designing new macromolecular architectures for many applications by finely tuning the structure of nickelocene monomers.”
The new work “is really something special,” adds Jens Müller of the University of Saskatchewan. Ring-opening polymerization of strained sandwich compounds is an elegant and well-established method to access metal-containing polymers, Müller notes. But even after years of investigation, it’s fascinating that this chemistry “is still good for a surprise,” he says. “Beyond the unprecedented reversible polymerization, the polymer itself is exciting as a rare case of a magnetic polymetallocene, an area that is in its infancy.”