Apr 17 2017
 A new surface coating developed by Kripa Varanasi and his team causes water to bead up on the inner surface of a pipe rather than spreading out. This prevents the formation of ices that could lead to a clog in an oil pipeline or well. CREDIT: Image courtesy of the researchers.
A new surface coating developed by Kripa Varanasi and his team causes water to bead up on the inner surface of a pipe rather than spreading out. This prevents the formation of ices that could lead to a clog in an oil pipeline or well. CREDIT: Image courtesy of the researchers.
On 21 April 2010, when there was a disastrous explosion and blowout in the Deep water Horizon oil rig which led to the worst ever oil spill, the operators of the well anticipated that the leak can be blocked within just a few weeks. On 9 May 2010, the operators triumphantly lowered a 125-ton containment dome on top of the broken wellhead. If that step would have been successful, the leaking oil would have been funneled into a pipe carrying the oil to a tanker ship above, thereby blocking the ongoing leakage which rendered the spill highly catastrophic. However, why the containment did not work as anticipated?
The reason was the presence of an icy mixture of methane and frozen water, termed as methane clathrate. Due to the high pressure and low temperatures at the sea floor, the slushy mixture aggregated in the inner side of the containment dome and obstructed the outlet pipe, thus hindering it from redirecting the flow; but for the presence of methane clathrate, the containment would have worked, and unstoppable leakage and large-scale ecological devastation for four months would have been averted.
At present, a group of scientists from MIT have found out a solution for averting such catastrophic outcomes of an oil spill. The invention can also prevent blockages in the inner side of oil and gas pipelines that can cause expensive shutdowns for clearing a pipe or, even worse, cause pipeline rupture due to build-up of pressure.
The innovative technique for preventing the icy build-up has been reported in a paper by associate professor of mechanical engineering Kripa Varanasi, postdoc Arindam Das, and recent graduates Taylor Farnham SB ’14 SM ’16 and Srinivas Bengaluru Subramanyam PhD ’16. The paper has been published in ACS Applied Materials and Interfaces.
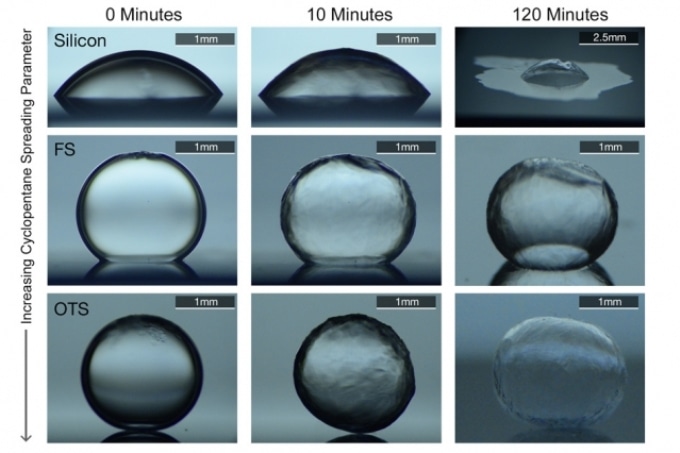
The solution offered by the new system is to coat the inner side of the pipe with a layer of a material that fosters the spreading of a water-barrier layer along the inner surface of the pipe. The researchers discovered that the barrier layer has the ability to effectively arrest the adhesion of water droplets or ice particles to the wall, thus preventing the build-up of clathrates that slow down or block the flow.
New coating could prevent pipeline clogging
Researchers at MIT have developed a coating that could stop the buildup of hydrate ices that slow or block oil and gas flow. These hydrates are potentially explosive and are largely responsible for the initial failure to contain the oil spill that rocked the Gulf of Mexico in 2010. (Melanie Gonick/MIT)
In contrast to potentially polluting and costly techniques such as depressurization, heating of pipe walls, or applying chemical additives, which were used earlier, the new technique is entirely passive—once it is commissioned, the technique avoids the need for further addition of material or energy. The liquid hydrocarbons that already occur in the flowing petroleum are attracted by the coated surface, thus forming a thin surface layer with the ability to naturally repel water. This primarily avoids the adherence of ices to the wall.
Varanasi stated that the prevalent measures for preventing blockage, called as flow assurance measures, “are expensive or environmentally unfriendly,” and at present, the application of these measures “runs into the hundreds of millions of dollars” every year. In the absence of these measures, the build-up of hydrates can reduce the flow rate, reducing the income. Moreover, stated Varanasi, if the hydrates cause blockages, then that “can lead to catastrophic failure.” “It’s a major problem for the industry, for both safety and reliability.”
According to Das, the lead author of the paper, the problem can become further complicated as methane hydrates that are abundant in various locations such as continental shelves are considered to be a new potential fuel source, if techniques for extracting them are devised. “The reserves themselves substantially overshadow all known reserves [of oil and natural gas] on land and in deep water,” stated Das.
However, deposits such as these might be more vulnerable to plug formation and freezing when compared to the prevalent oil and gas wells. Putting an end to such icy build-ups crucially depends on preventing the adherence of the very first particles of clathrate to the pipe: according to Farnham “Once they attach, they attract other particles” of clathrate, and the build-up starts to occur very quickly. “We wanted to see how we could minimize the initial adhesion on the pipe walls.”
The technique is identical to the one used in a company established by Varanasi to enable commercial production of research from his lab, related to coatings for containers that prevent the contents such as ketchup, honey, paint, or agrochemicals from being adhered to walls of a container. The system includes two steps: the first one is to form a textured coating on the walls of the container, and the next is to add a lubricant that gets trapped in the texture and averts adherence of the contents.
Varanasi explained that the new pipeline system is identical to that, but here “we are using the liquid that’s in the environment itself,” in contrast to applying a lubricant to the surface. He further stated that the most significant property with respect to clathrate formation is the occurrence of water; therefore, the build-up of clathrates can be prevented by keeping the water away from the pipe wall. Moreover, the liquid hydrocarbons in the petroleum get adhered to the wall due to the chemical affinity of the surface coating, thereby effectively keeping water away.
“If the oil [in the pipeline] is made to spread more readily on the surface, then it forms a barrier film between the water and the wall,” stated Varanasi. The researchers reported that in the investigations conducted in the laboratory using a proxy chemical for methane (as actual methane clathrates form only under high-pressure conditions difficult to reproduce in lab), the system was found to be highly effective. “We didn’t see any hydrates adhering to the substrates,” stated Varanasi.
The Italian energy company Eni S.p.A. funded the study, through the MIT Energy Initiative.