May 18 2017
For the first time, Researchers from the Helmholtz-Zentrum Berlin (HZB) have developed a nanomaterial produced from nanoparticles of a titanium oxide compound—namely, Ti4O7 (having an exceptionally large surface area)—and analyzed its usage as a cathode material in lithium-sulfur batteries.
The new nanomaterial is extremely porous and has a high storage capacity that stays almost constant over numerous charging cycles.
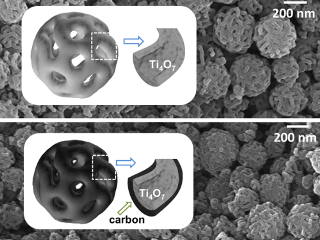 The porous structure of the nanoparticles is visible under the electron microscope. Credit: HZB.
The porous structure of the nanoparticles is visible under the electron microscope. Credit: HZB.
Now lithium batteries are considered to be one of the most ideal solutions for storing electrical power in a small space. During the discharge cycle, the lithium ions in the batteries travel from the anode to the cathode. In general, the cathode and anode comprise of heavy-metal compounds that are toxic as well as costly.
An impressive substitute to the lithium battery is the lithium-sulfur battery in which the cathode comprises a readily available and economic material, that is, sulfur, instead of heavy metals. When lithium ions from the anode travel toward the cathode during the discharge cycle, lithium sulfide (Li2S) is formed through the reaction between lithium and sulfur through different intermediate lithium polysulfides. During the charging cycle, lithium polysulfides get dissolved, resulting in a decline in the battery’s capacity during multiple charging cycles because of the so-called “shuttle effect.” Therefore, Scientists worldwide are striving to enhance the cathode materials that would be able to chemically or physically confine or encapsulate polysulphides, such as with nanoparticles formed of titanium dioxide (TiO2).
Ti4O7-nanoparticles with interconnected pore structure
Led by Professor Yan Lu, the HZB Researchers have at present developed a cathode material that is quite efficient. Even in this case, nanoparticles provide confinement of the sulphur. Yet, instead of titanium dioxide, they comprise of Ti4O7 molecules arranged on a porous spherical surface. These porous nanoparticles bind polysulphides substantially more strongly than the usual TiO2.
We have developed a special fabrication process to generate this complex, three-dimensionally interconnected pore structure.
Professor Yan Lu, Helmholtz-Zentrum Berlin
Yan Lu initially developed a template formed of a matrix of tiny polymer spheres with porous surfaces. The template was prepared in additional steps and then immersed in a solution of titanium isopropoxide. A layer of Ti4O7 formed on the porous spheres and remains after thermal treatment, which decomposes the underlying polymer. When compared to other cathode materials formed of titanium oxides, the Ti4O7 nanosphere matrix has an exceptionally large surface area. Just 12 g of the material is adequate to cover a football field.
Function decoded at BESSY II
X-ray spectroscopy measurements (XPS) at the CISSY experiment of BESSY II demonstrated that sulfur compounds bind strongly to the surface of the nanomatrix.
High specific capacity
This also accounts for the high specific capacity per gramme (1219 mAh) at 0.1 C (1 C = 1675 mA g-1). The specific capacity was also found to be reduced only a little during repeated discharge/charge cycles (0.094% per cycle). In contrast, the specific capacity of cathode materials formed of TiO2 nanoparticles is 683 mAh/g. In order to enhance the conductivity of the material, a supplementary coating of carbon can be applied to the nanoparticles. This process ensures that the highly porous structure stays unaffected.
Upscaling is feasible
We have been working to improve the repeatability of this synthesis for over a year. Now we know how to do it. Next, we will work on fabricating the material as a thin-film.
Professor Yan Lu, Helmholtz-Zentrum Berlin
Moreover, of interest is the fact that the research work that was successful in the lab can be shifted to commercial production as well because the entire line of processes, starting with the colloid chemistry up to the thin-film technology, are scalable.