Sep 22 2017
New lithium electrodes coated with indium could lay the foundation for longer-lasting, more powerful, rechargeable batteries.
As reported by American scientists in the journal Angewandte Chemie, the coating offers a more uniform deposition of lithium when charging, inhibits detrimental side-reactions between the electrolyte and electrode, and extends storage in the lithium anode through alloying reactions between indium and lithium. Their success is a result of the good diffusion of lithium ions along the interfacial layer.
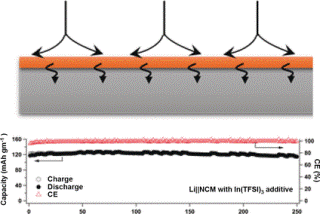 Image Credit: Wiley
Image Credit: Wiley
Usually, present lithium ion batteries have graphite anodes that store lithium when the batteries are charged. A remarkable alternative is presented by batteries with metallic anodes, such as lithium metal, which assure significantly higher storage capacity. Despite their successful implementation, a major drawback has been the uneven deposition of the metal during the charging process, which resulted in the formation of dendrites. After prolonged use of the battery, these dendrites can grow so wide that they short-circuit the battery. Additionally, there are adverse side-reactions between the electrolyte and the reactive metal electrodes, which considerably lower the lifetime of the batteries. An ideal solution would be the formation of a passivating, stable layer that prevents further contact; however, it is impossible because of the constant contraction and expansion of the electrode upon discharging and charging. This damages the layer and introduces the metal to the electrolyte for more reactions. Physical barriers or artificial films are some of the other approaches.
At present, an innovative alternative has been proposed by the researchers working with Ravishankar Sundararaman at Rensselaer Polytechnic Institute (Troy, USA) and Lynden A. Archer at Cornell University (Ithaca, USA). They created indium coatings on lithium by using straightforward electroless ion-exchange chemistry. All it requires is only a simple immersion in a special indium salt solution. Some of the indium is deposited on the surface of the lithium electrode as metal and the lithium ion concentration in the electrolyte increases at the same time.
The addition of small amounts of the indium salt to the electrolyte makes the indium layer self-healing and uniform when the electrode is in use. It remains in place during charge/discharge cycles, its chemical composition does not change, and side-reactions are prevented. In addition, dendrites are also removed, leaving the surface compact and smooth.
The researchers were able to prove the success of their method by using computer modeling: lithium ions are extremely loosely bound to the indium coating. They form an alloy with the indium, which enables them to move very quickly over the surface before crossing it, following which they are deposited on the underlying lithium electrode. In complete cells with commercial cathodes, these latest indium–lithium hybrid electrodes were stable over more than 250 cycles, preserving about 90% of their capacity.