Feb 5 2018
When a fragile surface needs a rock-hard, super-thin, bonded metal coating, standard manufacturing processes come up short. However, Cold Gas Dynamic Spray (CGDS) can do just that - with a big caveat. CGDS is extremely versatile but it is also extremely difficult to predict aspects of the process. Presently, a temperature-based 3D model by Professor Tien-Chien Jen from the University of Johannesburg begins to unlock the mysteries of the CGDS film-growing process in the particle deposition zone.
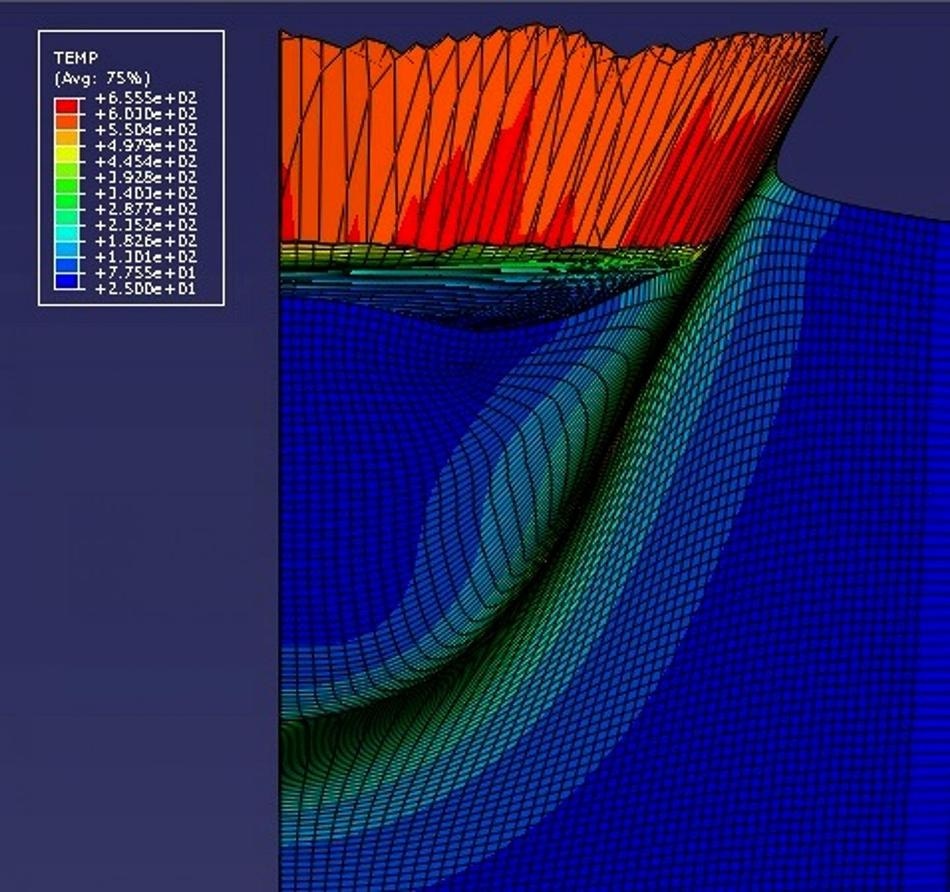 The CGDS deposition zone is 3-D modeled to show the effects of temperature for the first time, by Professor Tien-Chien Jen from the University of Johannesburg. In this image, the model predicts an orange/red "splash" of aluminum substrate just after a 5-micron copper particle has impacted it at 700 meters per second. (Image credit: Hong-Shen Chen, University of Johannesburg)
The CGDS deposition zone is 3-D modeled to show the effects of temperature for the first time, by Professor Tien-Chien Jen from the University of Johannesburg. In this image, the model predicts an orange/red "splash" of aluminum substrate just after a 5-micron copper particle has impacted it at 700 meters per second. (Image credit: Hong-Shen Chen, University of Johannesburg)
The model is the very first to connect the dots between energy transformation, particle impact velocity and temperature rise in the particle impact zone; in three dimensions.
CGDS is already employed extensively to repair or manufacture metal parts for large passenger airliners and also for military equipment and mobile technology.
In the process, a de Laval nozzle sprays micron-sized metal particles over a short distance, usually at 25 mm, at a metal or polymer surface. The particles hit the surface at speeds ranging from 300 m per second to 800 m per second - as a frame of reference, the speed of sound is 343 m per second.
CGDS has the best temperature range of all industrial spraying processes presently used and saves energy, since no heating is carried out.
Manufacturing Mystery
However, if individuals have a 5 μm copper particle, how fast should it arrive at the deposition zone on aluminum if they have not used this combination before? Or if they select a new metal for the particles, and a new metal for the surface, how do they even start guessing what size the particles should be and at what speed they should impact the surface to provide a well-bonded coating?
It should be noted that, the CGDS bonded coating should come without crystallization, evaporation, residual stresses or various other thermal damages - some of the big reasons CGDS is employed in the first place. These questions can indeed have huge financial implications for the manufacturing machines on the factory floor.
Physics Still Out
Why CGDS bonds metal particles to a substrate surface has challenged understanding since its invention in the 1980s by the military, states Jen, Professor in the Department of Mechanical Engineering Science at the University of Johannesburg.
At first, the military used CGDS to repair spare parts in the middle of nowhere. Then other industries realized you can use it on very fragile surfaces as well. You can create a new bonded surface only a few microns thick, or keep spraying until you have a 10mm coating. Once you've filled in the cracks or holes, you can machine the part to have its exact dimensions again, because the GDS bonded coating can be harder than the titanium or vanadium the part is made from.
Chien Jen, Department of Mechanical Engineering Science
The CGDS coating can be this hard due to the compressive stresses developed when the particles impact the surface. The stresses increase the metal fatigue life, he states. This is similar to what takes place in shot-peening, an industrial process very much like CGDS, but employing "balls" a few millimeters in diameter to impact a surface.
"CGDS is used for very high-cost manufacture and repair, but there is no comprehensive, realistic model describing the physics of the entire process," says Jen.
3D with Splashing
In CGDS, engineers discuss about two zones. The first is the flight-zone between the spray nozzle and the surface to be sprayed. This zone was modeled by Jen in a 2005 research article in the International Journal of Heat and Mass Transfer.
The second zone is the deposition zone, where the sprayed articles influence the surface. This zone is described by the new 3D model.
Previous two dimensional models have made attempts to solve the puzzle involving CGDS bonding, but these have a severe limitation. When a 2D model is extended to 3D, it leads to a 'horizontal cylinder' descending towards the surface being sprayed.
"Unfortunately, a descending cylinder cannot model realistically enough what happens to discrete ball-shaped particles 'splashing' down in the substrate surface," says Jen.
As industry knows, the speed (velocity) the particle arrives at the substrate is important. Too slow, and it will only bounce off. Too fast, and it could pass like a bullet via a thin substrate.
The new model animates in 3D a single spherical particle 'falling down' into the substrate metal. The substrate 'splashes up', and then the particle and substrate bond. The substrate 'splashing' looks like milk splashing up when something falls into the cat's bowl. This is called jetting behavior in industry.
Chien Jen, Department of Mechanical Engineering Science
Cold Metal, Temperature Rise
The model uses a number of parameters describing the nature of the particle and the surface: thermal conductivity, specific heat, density melting point, Poisson's ratio, elastic modulus, Johnson-Cook damage and Johnson-Cook plasticity.
It is the first to predict in 3D how the average temperature of the particle impact zone will rise and then subside, based on the impact velocity and size of the particle. The model was featured in the Journal of Thermal Spray Technology.
Just Fast Enough to Melt
"For this 3D model, we went with the hypothesis that a metal particle has to bond with the substrate at 60% of its melting temperature, to create a strong new surface without damaging the substrate," says Professor Jen.
As an example, copper (Cu) comprises of a melting temperature of 1083 oC and 60% of that is 650 oC. Hence, the hypothesis says that a 5 μm copper particle impacting an aluminum substrate surface will have to be fast enough so that the average impact zone temperature goes up to at least 650 C, and not much more, for good bonding to take place. According to the model, that critical impact velocity range is between 700 and 800 m per second.
Supersonic Energy Transformation
When a copper particle passes at a supersonic speed and hits an aluminum surface, its moving (kinetic) energy is transformed into heat (thermal) energy, says Prof Jen. This is based on the impact speed of the particle.
"The heat makes the particle and the impact zone 'soft and sticky', similar to melted cheese. The particle changes into a 'soft blob' that fills in the 'impact crater' in the substrate surface. At the same time, friction develops between the blob and the crater surface, which is critical to the bonding process," he says.
"The friction 'grabs' the blob, and it sinks into the substrate surface. As the blob sinks down, the molten substrate around the particle 'splashes up' in typical jetting behavior. When the jetting settles down, the bond between particle and surface is completed," says Jen.
Model vs the Real World
The model, despite being limited, holds up in experimental results with copper particles sprayed onto an aluminum surface.
"When the impact velocity is within the range predicted by the model for a particle size, sufficient bonding temperature is reached and a strong CGDS coating is created. As an example, we set up our CGDS equipment in the laboratory for copper particles with an average size of 5 micron, carried by nitrogen, and impact velocity in the range of 700 to 800 meters per second deposited downwards on aluminum.
"The model predicts that at about 750 meters per second impact velocity, the critical bonding temperature of 650 degrees Celsius will be attained in the particle impact zone. In line with that prediction, we obtained excellent CGDS bonded coatings," he says.
"However, as also predicted by the model, we found with our laboratory setup that when the particle impact velocity is not within the critical range, insufficient bonding temperature is reached. This can result in poor surface coating with loosened powders and scrapping surface, which don't meet manufacturing quality standards," says Jen.
Grand Challenge Remains
The single-particle single-layer 3D model will be further extended into a multi-particle, multi-layer model in follow-up projects.
This 3D model is the first to describe how the temperature of the impact zone influences particle deposition. However, realistically modeling the deposition zone in CGDS remains a grand challenge to solve. In real-world conditions, particles are not of uniform size or shape, and travel at different velocities and angles. So a more complete model will have to accommodate ranges, or distributions, of all of these parameters.
Chien Jen, Department of Mechanical Engineering Science