May 17 2018
A team of researchers has demonstrated that high oxide-ion conductivity is caused by the overbonding of channel oxygens in La-rich apatite-type lanthanum silicates and not by the presence of the interstitial oxygens.
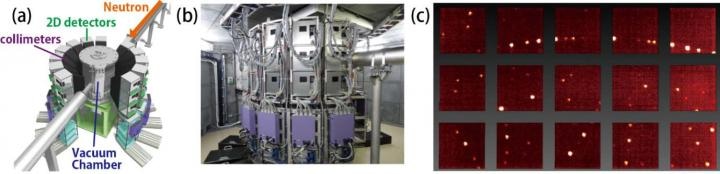 (a) A schematic figure and (b) a photograph of the SENJU diffractometer installed at the J-PARC facility. (c) Measured single-crystal neutron diffraction images. (Image credit: J-PARC)
(a) A schematic figure and (b) a photograph of the SENJU diffractometer installed at the J-PARC facility. (c) Measured single-crystal neutron diffraction images. (Image credit: J-PARC)
The study was performed by researchers from Tokyo Institute of Technology (Tokyo Tech); National Institute of Technology, Niihama College; Nagoya Institute of Technology; Japan Atomic Energy Agency; and Neutron Science and Technology Center, The Comprehensive Research Organization for Science and Society (CROSS). This notion of "high oxide-ion conductivity by overbonding" paves the way for developing better ion conductors, which could find applications in environmental protection and energy conversion.
Solid oxide electrolytes are used in a variety of applications such as oxygen membranes, solid oxide fuel cells (SOFCs), gas sensors, and catalysts and as a result, they have been studied extensively. To decrease the SOFCs’ operation temperature, electrolytes with high oxide-ion conductivity at temperatures less than 600 °C are needed. In 1995, Professor Susumu Nakayama at National Institute of Technology, Niihama College discovered the very high oxide-ion conductivity in the intermediate temperature range less than 600 °C, prompting many scientists to examine the phenomenon’s structural origin.
It was assumed that interstitial oxygens were responsible for the high oxide-ion conductivity of apatite-type materials, but in this new research, Dr. Kotaro Fujii at Tokyo Institute of Technology (Tokyo Tech), Professor Masatomo Yashima, and their coworkers have demonstrated that apatite-type materials lack oxygen interstitials but contain Si vacancies. Professor Koichiro Fukuda at Nagoya Institute of Technology originally proposed the Si vacancies in the materials.
Using the SENJU diffractometer installed at MLF, J-PARC facility (Figure 1), the researchers performed single-crystal neutron diffraction studies and precisely established the crystal structures of the apatite materials La-rich La9.565(Si5.826□0.174)O26 (□ represents Si vacancy) and La9.333Si6O26 including atomic displacement parameters, occupancy factors, and spatial distributions of oxygen atoms. The team also calculated the oxide-ion conductivity and density of the two materials. La9.565(Si5.826□0.174)O26 was selected in this analysis due to its high oxide-ion conductivity.
Through structure analyses using the diffraction data, the scientists discovered Si vacancies but no interstitial oxygens. At the O4 site in the apatite channel, they also found a larger positional disorder of the oxide ion as compared to the basic La9.333Si6O26 material (Figure 2). For the oxide-ion conduction along the c axis, the lower activation energy was found to be primarily responsible for the higher oxide-ion conductivity of the La9.565(Si5.826□0.174)O26 material when compared to the La9.333Si6O26 one. When compared to La9.333Si6O26, the surplus La produced the overbonding of the O4 oxide ion in La9.565(Si5.826□0.174)O26 resulting in higher oxide-ion mobility and conductivity of the La9.565(Si5.826□0.174)O26 material with Si vacancies (Figure 2). The presence of Si vacancies in La9.565(Si5.826□0.174)O26 was supported by density measurements through the Archimedes method.
The scientists therefore proposed that surplus La cations are the main reason for overbonded channel oxygens along the c axis, leading to highly anisotropic atomic displacement and thus high oxygen mobility. Therefore, this new concept of "high oxide-ion conductivity by overbonding" could prove useful for developing better ion conductors.