Dec 14 2018
Traditional lithium-ion batteries, for example, those largely used in notebooks and smartphones, have already reached their performance limits.
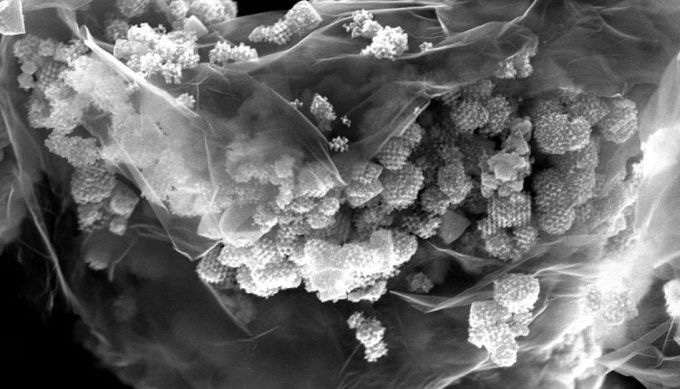 HRSEM picture of a 2D/3D nanocomposite based on graphene. (Image credit: Freddy Kleitz/Universität Wien; Glaudio Gerbaldi/Politecnico di Torino, CC-BY-NC)
HRSEM picture of a 2D/3D nanocomposite based on graphene. (Image credit: Freddy Kleitz/Universität Wien; Glaudio Gerbaldi/Politecnico di Torino, CC-BY-NC)
Freddy Kleitz, materials chemist from the Faculty of Chemistry of the University of Vienna, and international researchers have created an innovative nanostructured anode material for lithium-ion batteries, which increases the capacity and cycle life of the batteries. The material, which is based on a mesoporous mixed metal oxide coupled with graphene, could offer a novel strategy to make optimal use of batteries in large devices like electric or hybrid vehicles. The research has been published as the cover story of the latest issue of Advanced Energy Materials.
Due to their high energy density, zero memory effect, and extended cycle life, lithium-ion batteries are not only the most widespread energy storage devices for mobile devices but also bearers of hope for electro-mobility. Scientists are on the search of innovative types of active electrode material to push the batteries to the subsequent level of high performance and durability, and to render them better usable for large devices. “Nanostructured lithium ion battery materials could provide a good solution,” stated Freddy Kleitz from the Department of Inorganic Chemistry–Functional Materials of the University of Vienna, who together with Claudio Gerbaldi, leader of the Group for Applied Materials and Electrochemistry at the Politecnico di Torino, Italy, is the main author of the study.
The 2D/3D nanocomposite developed by the two researchers and their colleagues is based on a mixed metal oxide and graphene; hence, it significantly improves the electrochemical performance of lithium-ion batteries.
In our test runs, the new electrode material provided significantly improved specific capacity with unprecedented reversible cycling stability over 3,000 reversible charge and discharge cycles even at very high current regimes up to 1,280 milliamperes.
Freddy Kleitz, Head, Department of Inorganic Chemistry–Functional Materials, University of Vienna.
The existing lithium-ion batteries lose their performance after nearly 1000 charging cycles.
New recipe
Traditional anodes are usually made of carbon material such as graphite.
Metal oxides have a better battery capacity than graphite, but they are quite instable and less conductive.
Freddy Kleitz, Head, Department of Inorganic Chemistry–Functional Materials, University of Vienna.
The scientists discovered a technique to make optimal use of the positive features of both compounds. They created a new class of electrode active materials, which is based on a mixed metal oxide and the highly conductive and stabilizing graphene, exhibiting exceptional properties in contrast to a majority of the transition metal oxide nanostructures and composites.
As an initial step, using a newly designed cooking method, the scientists were able to homogenously mix copper and nickel in a controlled way to accomplish the mixed metal. Using nanocasting—a technique for producing mesoporous materials—they developed structured nanoporous mixed metal oxide particles, which have an extremely high active reaction area for the exchange with lithium ion from the electrolyte of the battery due to their extensive network of pores. Then, the researchers used a spray drying technique to wrap the mixed metal oxide particles tightly with thin graphene layers.
Simple and efficient design
From an environmental perspective, using lithium-ion batteries for e-mobility is regarded to be difficult, for instance, because of their raw material-intensive production. Small batteries with the ability to store the maximum possible energy last as long as possible and are not very cost-intensive to produce could advance their application in large-scale devices. “Compared to existing approaches, our innovative engineering strategy for the new high-performing and long-lasting anode material is simple and efficient. It is a water-based process and therefore environmentally friendly and ready to be applied to industrial level,” concluded the study authors.