Feb 18 2019
Chemists at LMU have found a mechanism that permits molecules to diffuse speedily on the already crowded surface of a solid-state catalyst—a critical capability, mainly for efficient catalysis under industrial settings.
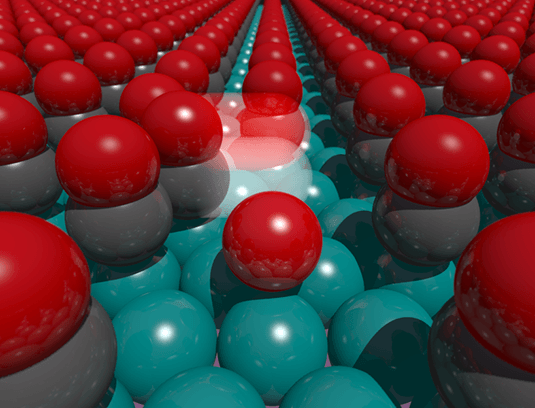 Fluctuations in the arrangement of the carbon monoxide molecules enable an oxygen atom to rapidly traverse an already densely occupied surface of a catalyst. (Image credit: Bild: Ann-Kathrin Henß)
Fluctuations in the arrangement of the carbon monoxide molecules enable an oxygen atom to rapidly traverse an already densely occupied surface of a catalyst. (Image credit: Bild: Ann-Kathrin Henß)
The use of efficient catalytic agents is what primarily makes many technical procedures possible. Certainly, synthesis of over 80% of the products made in the chemical industry requires the input of particular catalysts. A majority of these are solid-state catalysts, and the reactions they make possible occur between molecules that adsorb to their surfaces. The precise properties of the catalyst allow the starting molecules to interrelate and quicken the reaction between them, without changing or consuming the catalyst itself. However, efficient catalysis also necessitates efficient mixing, so reactants must be able to diffuse laterally on the surface of the catalyst to maximize the opportunity of experiencing the preferred reaction. Under the settings used in industrial processes, however, the surface of the catalyst is normally so thickly packed with adsorbed particles that it has been vague how molecules could efficiently diffuse at all.
Scientists led by Professor Joost Wintterlin at the Department of Chemistry at LMU have currently demonstrated that, although reactants spend time virtually imprisoned on the surface of the catalyst, local fluctuations in occupancy frequently provide chances to alter positions. The new findings have been published in the leading journal Science.
So as to gain an understanding into the molecular processes that occur on a solid-state catalyst, Wintterlin and colleagues employed scanning tunneling microscopy (STM) to track the mobility of individual oxygen atoms on a ruthenium (Ru) catalyst that was tightly packed with adsorbed carbon monoxide (CO) molecules.
We chose this system because the oxidation of CO to CO2 on metals belonging to the platinum group is a well-studied model for solid-state catalysis generally.
Joost Wintterlin, Professor, Department of Chemistry, LMU.
However, conventional scanning tunneling microscopy would have been incapable of capturing the surface dynamics of this reaction system. But the researchers were successful in improving the rate of data acquisition, at last, reaching rates of up to 50 images per second—sufficiently high to create videos of the dynamics of the particles on the catalyst.
The STM images showed that the oxygen atoms are totally hemmed in by triangular cages created by CO molecules adsorbed to the surface of the Ru catalyst. Examination of the videos revealed that single oxygen atoms can only hop between three positions created by the interstices of the Ru atoms.
But, to our surprise, we also observed that an atom can escape from its cage, and suddenly begins to diffuse through the carbon monoxide matrix at a rate that is almost as high as if it were on a completely empty surface.
Ann-Kathrin Henß, Study First Author, LMU
In partnership with Professor Axel Groß of the Institute of Theoretical Chemistry at Ulm University, the Munich team was able to connect this occurrence with fluctuations in the local density of the CO on the surface, which create regions in which the molecules are quite tightly packed together. When such a fluctuation takes place near an oxygen atom, the latter can escape from its cage, and travel to a new position. Actually, this “door-opening mechanism” unlocks diffusion pathways so fast that the movement of the oxygen atoms via the matrix is not greatly obstructed. This explains why they can practically always find a new binding partner for the reaction enabled by the catalyst.