Mar 6 2019
The two main reactive forms of nitrogen are ammonia and nitric acid, which are extensively used in human life and production. In 2017, the global production of NH3 and HNO3 was calculated to be 150 and 50 million metric tons, respectively.
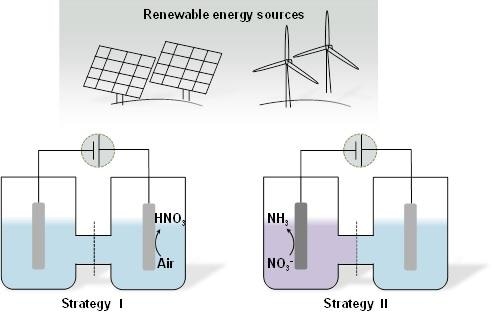 Schematic illustration for as-proposed Strategy I and Strategy II. (Image credit: Science China Press)
Schematic illustration for as-proposed Strategy I and Strategy II. (Image credit: Science China Press)
Nowadays, commercial nitric acid is synthesized by the oxidation of ammonia, which is known as Ostwald method. Ammonia is produced by the traditional Haber-Bosch process. Both of these industrial processes are energy-inefficient and release a huge amount of greenhouse gas. Or else, the conventional centralized mode of production and transformation always uses plenty of energy and therefore results in the waste of fossil fuels.
In this context, looking for new solutions that enable energy-efficient and environment-friendly production of HNO3 and NH3 at distributed sources is an emergent need. In a new study reported in National Science Review, the Zhang group at the Department of Chemistry at Tianjin University has exhibited the most recent advances in electrochemical synthesis of ammonia and nitric acid.
The potentially alternative approaches they suggested are exploiting copious air and waste nitrate combined electrocatalytic process to produce HNO3 and NH3. Notably, the focus of current nitrogen fixation is on preparing NH3 by reducing pure dinitrogen, which is formed by the energy-intensive air-separation process. In addition, it is also limited by the supplementary hydrogen evolution reaction.
Hence, the Zhang group projected two new strategies. Strategy I involves the electrocatalytic oxidation of N2 to HNO3 by using air as the nitrogen source, and Strategy II involves the electrochemical reduction preparation of ammonia from residual nitrate ions (NO3−) contamination in water.
Platinum foil with a Faradaic efficiency of 1.23% at +2.19 V vs reversible hydrogen electrode (RHE) is used as the catalyst for nitrogen oxidation, and Co3O4 nanorod array catalyst is used for nitrate reduction to ammonia with ammonia selectivity of 33.6% at −0.65 V vs RHE. Moreover, the isotope labeling experiments prove the origin of nitrogen for acquired nitric acid and ammonia. These discoveries open up new possibilities for manipulating the reactive nitrogen species in an “economic” and “clean” manner, particularly when the electrocatalysis reaction is driven by renewable energy.