Mar 8 2019
Hydrogen is a highly desirable as well as highly explosive energy carrier, which needs lightweight, safe, and inexpensive storage and transportation systems. Currently, researchers at the Weizmann Institute of Science, Israel, have created a chemical storage system using simple and plentiful organic compounds.
 (Image credit: Wiley-VCH)
(Image credit: Wiley-VCH)
As described in the journal Angewandte Chemie, the theoretical capacity of the liquid hydrogen carrier system is very high and it uses the same catalyst for the charging-discharging reaction.
Hydrogen holds a lot of energy, which can be turned into power or electricity. Water is the only combustion byproduct during this conversion. However, since hydrogen is a gas, it has a low energy density by volume. Thus, pure hydrogen is generally handled in its liquid form or pressurized state; however, steel tanks increase weight, and its discharge and usage become dangerous.
Besides tanks, hydrogen can also be enclosed and stored in a chemical reaction system. This is the fundamental way by which nature stores and uses hydrogen: In biological cells, finely adjusted chemical compounds merge and emit hydrogen to accumulate the chemical compounds required by the cells. Catalyzation of all these biological processes is done by enzymes.
Furthermore, potent catalysts that mediate hydrogen conversion have been synthesized in chemical laboratories. One example is the ruthenium pincer catalyst, which is a soluble complex of ruthenium with an organic ligand, produced by David Milstein and his teammates. They used this catalyst to analyze the capacity of a reaction system of simple organic chemicals to store and discharge hydrogen.
“Finding a suitable hydrogen storage method is an important challenge toward the ‘hydrogen economy’,” is how the authors of the publication described their inspiration. Easy loading and unloading schemes, safe chemicals, and a minimum possible volume are some of the criteria that must be met.
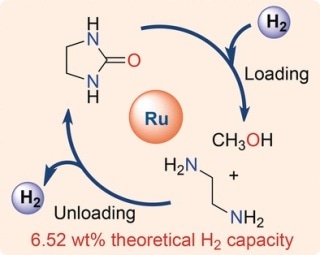
Milstein and his team identified such a system, containing the chemical compounds methanol and ethylenediamine. The reaction of two molecules releases pure hydrogen. A compound known as ethylene urea is the other reaction product. The theoretical capacity of this “liquid organic hydrogen carrier system” (LOHC) is 6.52% by weight, which is an extremely high value for a LOHC.
The researchers first established the hydrogenation reaction. This reaction involves the formation of liquid hydrogen carriers methanol and ethylenediamine from hydrogen gas and ethylene urea with 100% conversion when the ruthenium pincer catalyst was used.
After that, they analyzed the hydrogen release reaction, or the reaction of methanol with ethylenediamine. In this case, the yield of hydrogen was nearly 100%, but the reaction appeared to progress over intermediate stages and ended with an equilibrium of products. However, complete re-hydrogenation was possible, which made the authors to conclude that they had certainly created a fully rechargeable system for hydrogen storage. This system was developed using liquid organic compounds that are plentiful, inexpensive, easily handled, and not very dangerous.
The simple nature of the compounds and the high theoretical capacity are the advantages of this system. However, in order to be more efficient and greener, similar to a system in nature, reaction times have to be still shorter and temperatures must be lower. This requires examination of even “greener” catalysts.