Apr 30 2019
Pollutants and gases can be filtered from liquids and air using porous, crystalline materials, for example, metal–organic frameworks (MOFs). To partition these pores additionally and improve their sorption capacity, a team of researchers have formulated a rapid and versatile two-in-one synthetic strategy, merging metal coordination with the covalent chemistry of light elements. As mentioned in a research in the journal Angewandte Chemie, the new pore-space-partitioned material could be used as an extremely efficient adsorbent of ammonia.
 © Wiley-VCH
© Wiley-VCH
The structure of MOFs is a coordinative system of metals with organic linkers, which amasses a large and symmetric three-dimensional porous network. It is possible for gases to diffuse in and out of the pores. When in a MOF, gas molecules adsorb at adsorption areas provided by the linker molecules and the metal ions. However, small gas molecules such as acetylene, CO2, and ammonia do not need large pores to be captured, and it turns out that occasionally a denser network and more adsorption areas can improve the capacity of a MOF.
Thus, a team of researchers led by Pingyun Feng at the University of California, USA, tried to partition the pores using covalent ligands—spacer molecules that amass through chemical reactions. Partitioning has the extra advantage that it could render the MOF more stable. Uncertainty is one of the reasons why MOFs have not found extensive use yet, although they are a lot more efficient gas sorption materials than, for instance, activated carbon and zeolites.
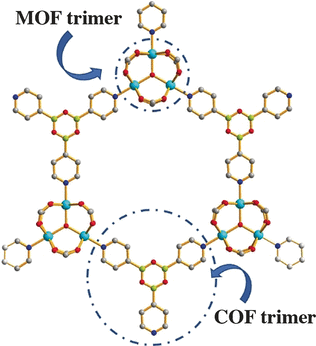
Feng’s team, including graduate student Yanxiang Wang, selected the aromatic molecule pyridine-4-boronic acid as a partitioning molecule. This is a rare ligand. It integrates two diverse light elements with complementary reactivity: boron is a Lewis acid and tends to trap agents with high electron density, while the pyridinic nitrogen is a Lewis base hunting for Lewis acids to react with. Under standard circumstances, these molecules would just attack each other and cause numerous non-targeted reactions.
However, this did not occur in this case as the researchers combined the pyridine-4-boronic acid reaction into the metal coordination reaction that amasses the MOF. Both covalent and coordinative reactions served synergistically and safeguarded the pyridine-4-boronic acid from side reactions. A trimer formed that fitted precisely into the hexagonal pores of the MOF. The outcome was a MOF with an incorporated covalent organic network, or “pore-space partitioned MOF”, offering a number of new sites for gas adsorption.
The researchers synthesized a number of these MOFs, each with a distinct mixture of metals and organic ligands. The new pore-space-partitioned MOFs exhibited improved gas uptakes than those that were unpartitioned. Furthermore, the exposed boron Lewis acid sites of the partitioning ligands allowed ammonia uptake having a high packing density. This research presents a progress in MOF synthesis and performance. Reactions that were not believed to be possible—for example, neat trimerization of a pyridineboronic acid—are realized and may result in very beneficial components.