May 6 2019
Graphene is well known as an amazing material. It comprises of pure carbon, with a thickness of only a single atomic layer. Although it is very strong, stable, as well as conductive, when it comes to electronic devices, it still has major demerits.
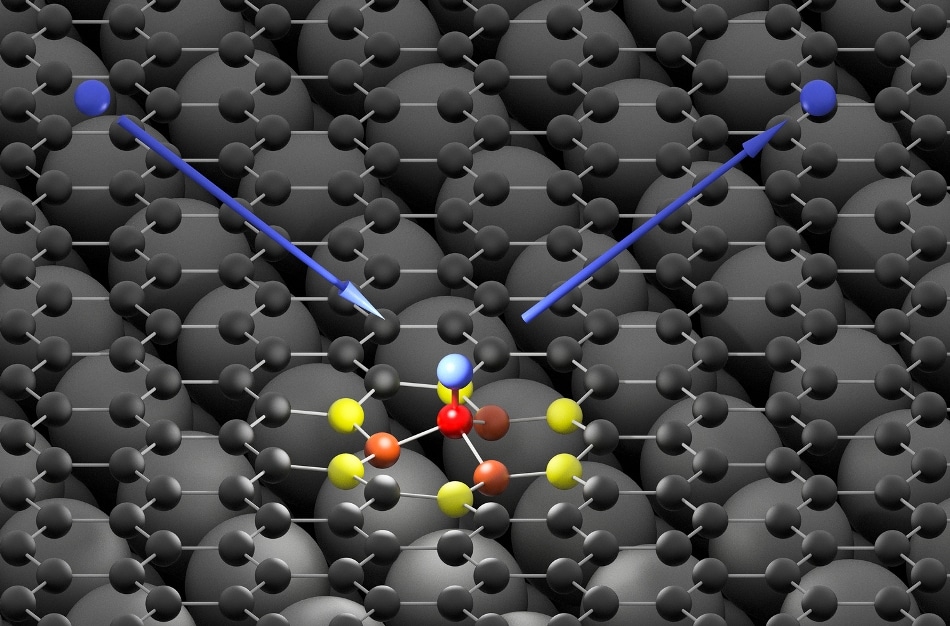 The hydrogen atom (blue) hits the graphene surface (black) and forms an ultra-fast bond with a carbon atom (red). The high energy of the impinging hydrogen atom is first absorbed by neighboring carbon atoms (orange and yellow) and then passed on to the graphene surface in the form of a sound wave (Image credit: Oliver Bünermann/Max Planck Institute for Biophysical Chemistry and University of Göttingen)
The hydrogen atom (blue) hits the graphene surface (black) and forms an ultra-fast bond with a carbon atom (red). The high energy of the impinging hydrogen atom is first absorbed by neighboring carbon atoms (orange and yellow) and then passed on to the graphene surface in the form of a sound wave (Image credit: Oliver Bünermann/Max Planck Institute for Biophysical Chemistry and University of Göttingen)
As graphene possesses no bandgap, it cannot be used as a semiconductor. A bandgap can be created by attaching hydrogen atoms to graphene. At present, scientists from Göttingen and Pasadena (United States) have developed an “atomic scale movie” demonstrating the chemical binding of hydrogen atoms to graphene in one of the fastest reactions studied until now (Science, April 25th, 2019).
The bombardment of graphene with hydrogen atoms was carried out by the global research team.
The hydrogen atom behaved quite differently than we expected. Instead of immediately flying away, the hydrogen atoms ‘stick’ briefly to the carbon atoms and then bounce off the surface. They form a transient chemical bond.
Alec Wodtke, Head of the Department of Dynamics at Surfaces, Max Planck Institute for Biophysical Chemistry
Alec Wodtke is also a professor at the Institute of Physical Chemistry at the University of Göttingen.
The researchers were surprised to find that the hydrogen atoms possessed more energy before collision with the graphene but not much left when they were scattered. Where does the energy lost by the hydrogen atoms on collision go?
Alexander Kandratsenka, researcher at Max Planck Institute for Biophysical Chemistry, Göttingen, together with coworkers at the California Institute of Technology, explained these amazing experimental observations by developing theoretical methods, which they simulated on the computer and later compared with their experiments. With these theoretical simulations, in line with the experimental observations, the scientists could reproduce the ultra-fast movements of atoms that form the transient chemical bond.
This bond lasts for only about ten femtoseconds—ten quadrillionths of a second. This makes it one of the fastest chemical reactions ever observed directly. During these ten femtoseconds, the hydrogen atom can transfer almost all its energy to the carbon atoms of the graphene and it triggers a sound wave that propagates outward from the point of the hydrogen atom impact over the graphene surface, much like a stone that falls into water and triggers a wave.
Alexander Kandratsenka, Researcher, Max Planck Institute for Biophysical Chemistry
The sound wave is the contributory factor that helps the hydrogen atom to bind more easily to the carbon atom than was anticipated by the researchers and predicted by the prior models.
The study outcomes offer a basic and new understanding of the chemical bonding. Moreover, they have attracted great attention from the industry. The binding of hydrogen atoms to graphene can form a bandgap, thereby making it a useful semiconductor and much more versatile in electronic devices.
Oliver Bünermann, project group leader at the University of Göttingen, stated that more effort has been invested in establishing and performing these experiments.
We had to carry them out in ultra-high vacuum to keep the graphene surface perfectly clean.
Oliver Bünermann, Project Group Leader, University of Göttingen
Also, the researchers used a huge number of laser systems to produce the hydrogen atoms prior to the experiment and to detect them following the collision. According to Bünermann, the success of the project was mainly because of the extremely good technical staff in the workshops at the Max Planck Institute for Biophysical Chemistry and at the University of Göttingen.